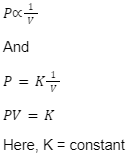Solid, liquid, gas, plasma, and the Bose-Einstein condensate are the five primary states of matter. Gases are the unique state among these five states. Their properties are simple to analyse. Gases, as we can see, follow a set of rules known as the gas laws. The behaviour of gases is described by these rules. That is, the values and relationships of temperature, pressure, and volume, etc.
Gas
Gas is one of the four basic states of matter (the others are solid, liquid, and plasma). A gas mixture like air. The gaseous state of matter resides between the liquid and plasma phases, and plasma is served as the upper temperature boundary for gases.
Kinetic Theory of Gases
The kinetic theory of gases is used to describe gas molecule behaviour. It is the study of gas molecules at the macroscopic level.
The following are the five postulates of the kinetic theory of gases:
Gas is made up of a large number of molecules that move about at random all the time.
The volume of gas molecules is negligible since the distance between them is greater than the size of the molecules.
Intermolecular interactions are minor as well.
Collisions between gas molecules and the container’s walls are always elastomeric.
The temperature affects the average kinetic energy of all the gas molecules.
Variables to Describe Behaviour of Gas
Variables describing behaviour of gas are given here.
Pressure
Volume
Temperature
Quantity
Due to the expansion of gas molecules, the volume of the gas expands as the temperature rises.
The volume of the gas decreases as the temperature drops due to the contraction of gas molecules.
The pressure of a gas rises as the temperature rises due to the expansion of gas molecules.
When the temperature drops, the pressure of the gas decreases due to the contraction of gas molecules.
To turn a gas into a solid or a liquid, the temperature or pressure of the gas must be extremely low or extremely high.
The pressure lowers as the quantity decreases, and the pressure rises as the quantity increases.
Ideal Gas
The molecules of an ideal gas are supposed to collide in almost perfect elastomeric collisions. As a result, even after collisions, the energy in the system remains constant. Furthermore, it is believed that the gas molecules are far apart, implying that they do not have a spatial constraint and can be regarded as point particles.
Ideal gas motion is constant and random along a straight line.
As the particles in gas are so small, the gas takes up very tiny space.
Absolute temperature is proportional to the average kinetic energy of gas particles.
The gas particles do not interact with each other. Particles solely collide with the container’s walls and each other on their own.
The gases, which are perfectly rigid spheres that are also exceedingly little, are made up of many of the same particles (atoms or molecules).
Gas molecules are called point masses because their volume is so small in contrast to the space between them.
Ideal Gas Law
The ideal gas law is a state equation describing ideal gases. This equation of state connects a gas’s pressure, volume, temperature, and mass, and it’s a great way to describe how gases behave under ideal conditions. For gases, this is the most common equation of state.
Boyle’s Law
The absolute pressure which is exerted by a given mass of an ideal gas is inversely proportional to the volume occupied by the gas when both the temperature and the amount of gas are kept constant. This is the statement of Boyle’s Law.
Boyle’s law is given as
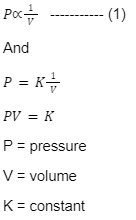
Charles’ Law
When the pressure of a gas is held constant, then the volume of the gas is proportional to its temperature. This the statement of Charles’ law.
It means that when temperature rises, the volume also rises and when temperature decreases, then also volume decreases.
Charles’ law is given as
V∝T ———- (2)
V = CT
Here, C = constant
Avagadro’s Law
According to Avogadro’s law, at constant temperature and pressure, the volume of a gas is directly proportional to the number of molecules present in that gas under constant temperature and pressure.
Avagadro’s law is given as
V∝n ———- (3)
Here,
n = number of moles
From equations (1), (2), and (3), we get

Therefore, the ideal gas equation is
PV=nRT
Here,
P = pressure
V = volume
T = temperature
R = gas constant
Conclusion
Gas is one of the four basic states of matter (the others are solid, liquid, and plasma).
The kinetic theory of gases is used to describe gas molecule behaviour.
Due to the expansion of gas molecules, the volume of the gas expands as the temperature rises.
When the temperature drops, the pressure of the gas decreases due to the contraction of gas molecules.
The pressure lowers as the quantity decreases, and the pressure rises as the quantity increases.
Boyle’s law is given as
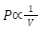
Charles’ law is given as
V∝T
Avagadro’s law is given as
V∝n
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out