Resonance is a mental exercise that depicts the delocalization of electrons inside molecules in the Valence Bond Theory of bonding. It entails creating numerous Lewis structures that, when assembled, reflect the molecule’s whole electrical structure. When a single Lewis structure cannot properly explain the bonding, resonance structures are employed; a resonance hybrid is defined as a mixture of potential resonance structures that describes the overall delocalization of electrons inside the molecule. In general, molecules with more resonance structures are more stable than those with less, and some resonance structures contribute more to a molecule’s stability than others – formal charges can help determine this.
Resonance: The Case of Ozone molecule (O3):
Resonance is a term used to describe delocalized electrons inside molecules or polyatomic ions when the bonding cannot be represented using a single Lewis formula. Several resonance structures depict a molecule or ion with such delocalized electrons. The Lewis skeleton’s nuclear skeleton The electron positions change, but the structure of these resonance structures remains the same. Such is the situation with ozone (O3), an oxygen allotrope with a V-shaped structure and a 117.5° O–O–O angle. Let’s get the conversation started by constructing the Lewis structure for ozone.
Since ozone has a V-shaped structure, one O atom is at the centre
There are 6 valence electrons in each O atom, for a total of 18 valence electrons.
When one bonding pair of electrons is allotted to each oxygen–oxygen connection, 14 electrons are absent.
We get three lone pairs of electrons on each terminal oxygen and have two electrons left over if we deposit three lone pairs of electrons on each terminal oxygen.
Both terminal oxygen atoms hold octets of electrons at this point. As a consequence, the ending two electrons are assigned to the centre atom.
There are merely 6 electrons in the core oxygen atom. Depending on the option we select, we will receive either There are only 6 electrons in the core oxygen atom. One lone pair on a terminal oxygen atom must be converted to a bonding pair of electrons—but which one? Depending on the option we select, we will receive either

Which is the right answer? In reality, neither is true. Both anticipate a single O–O bond and a double O=O bond. As you’ll see, the lengths of the bonds change if they’re of different kinds (one single and one double, for example). However, it turns out that the lengths between both O–O bonds are the same, 127.2 pm.
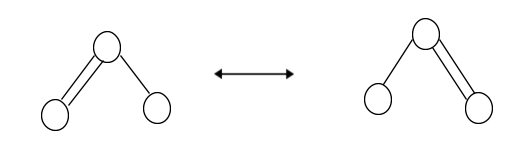
The double-headed arrow indicates that the molecule’s real electronic structure is an average of the two displayed, not that it oscillates between them.
The correct structure of the ozone molecule is the resonance hybrid of all the possible canonical structures.
While this is not exactly right because each resonance structure is distinct, it is a restriction of utilising the Lewis structure perspective to explain these compounds. Multiple resonance structures must be considered simultaneously for a more accurate representation of the molecule’s electron structure.
Rules for delocalisation and resonance:
Resonance structures should have the same amount of electrons; no electrons should be added or subtracted. (Count the number of electrons to see how many there are.)
Each resonance structure follows the Lewis Structures writing guidelines.
The Lewis Structures writing standards are followed for each resonance structure.
The structure’s skeleton cannot be altered (only the electrons move).
The number of lone pairs in a resonance structure must be the same.
Use correct arrows for indication:
To indicate resonance, a double headed arrow (↔) is utilised on both ends of the arrow between Lewis structures.
Equilibriums are denoted by double harpoons(⇌).
One electron is represented as a single harpoon(⇀) on one end.
The movement of two electrons is shown by a double headed arrow (→) on one end.
Identification of Resonance using formal charge:
While each resonance structure adds to the molecule’s total electronic structure, their contributions may not be equal. One way for determining the feasibility of a resonance structure and its relative significance among other structures is to assign formal charges to atoms in the molecules. In a covalent species, the formal charge on an atom is the total charge the atom would have if the electrons in all of the atom’s bonds were equally shared. In a covalent species, the formal charge on an atom is the net charge the atom would have if all of its bonds were nonpolar covalent bonds. Use the following formula to find the formal charge on a particular atom in a covalent species:
Formal Charge= (number of valence electrons in free orbital)− (number of lone-pair electrons)− 12 ( number bond pair electrons)
Rules of approximating stability of resonance structures:
The more covalent bonds there are , the more stable the system will be since more atoms will have full octets.
More stable is the structure with the fewest formal charges.
The structure with the least formal charge separation is the most stable.
The more electronegative atom in a structure with a negative charge will be more stable.
Positive charges on the most electropositive (least electronegative) atom are more stable.
Equivalent resonance forms have the same level of stability and contribute equally (e.g. benzene)
The Case of Benzene (C6H6): ![]()
![]()
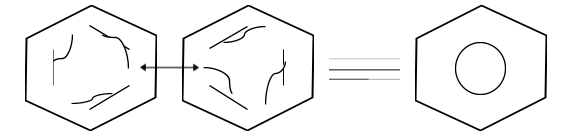
In organic chemistry, benzene is a particularly significant aromatic hydrocarbon. Its molecular formula is C6H6. The cyclic structure of benzene molecules is made up of alternating single and double bonds between nearby carbon atoms. Each carbon atom has a hydrogen atom linked to it. The two probable benzene resonance configurations are shown above.
The pi electrons are delocalized around the ring structure, which stabilises the benzene molecule. Each carbon-carbon bond has a bond order of 1.5 as a result of the delocalization, meaning that they are stronger than conventional C-C sigma bonds. The delocalization of pi electrons in the benzene resonance hybrid is described by a circle inside the hexagonal ring.
In the case of benzene, Kekule proposed two cyclohexatriene Kekule structures that, when combined, form the general structure as contributing structures. In the hybrid structure on the right, the hexagon substitutes three double bonds and represents six electrons in a collection of three molecular orbitals with a nodal plane in the molecule plane.
CONCLUSION:
Resonance is the phenomenon which can explain the stability of polyatomic ions and molecules. The resonance hybrid is the collection of all the canonical structures which can be formed by the delocalisation of electrons and bonds. Of all the canonical structures or resonating structures, the resonance hybrid is the most stable and has the least energy state.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






