Group 13 is the first group of p-block elements and is termed as the Boron family. The electronic configuration of group 13 elements is ns2np1. Boron is a non-metal, while aluminium, gallium, indium, and thallium are metals. Boron is a rare element found as Borax, kernite, and orthoboric acid in nature. Aluminium is an abundant metal found in the form of Bauxite in the earth’s crust.
Chemical properties of group 13 elements
Oxidation state and chemical reactivity
Boron atom is a small-sized atom, so it requires high three ionization enthalpies, therefore it cannot form cation (+3 ions), thus forming covalent compounds.
On moving down the group, the elements like Aluminium require less ionization enthalpy, thus forming Al3+ ion.
While going down the group, intervening d and f orbitals have poor shielding, thus increased effective nuclear charge which holds ns electrons tightly. So, they cannot form bonds easily, and only the p-orbital is involved in bonding.
Indium and Thallium have +1 and +3 oxidation states.
Compounds in the +1 oxidation state are more ionic than those in the +3 oxidation state.
The relative stability of +1 oxidation state: Al<Ga<In<Tl.
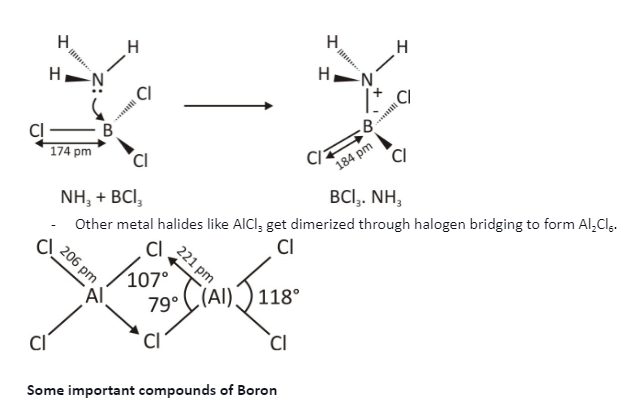
Electron deficient molecules of group 13 elements can accept a pair of electrons to achieve noble gas configuration, thus acting as Lewis acids. While going down the group, the tendency to form Lewis acid increases. It is a result of an increase in the size of the atom.
BCl3 accepts a lone pair of electrons from NH3 to form BCl3.NH3.
To become stable, AlCl3 forms a dimer.
The trivalent state of the compounds is covalent, thus hydrolyzing in water. For example, Aluminium chloride forms octahedral [Al(H2O)6]3- in acidified aqueous solution having SP3d2 on Al.
Reactivity towards air
In its crystalline form, Boron is unreactive, while Aluminium forms a thin layer of oxide on its surface to prevent the further attack of oxygen on it.
On heating in air, amorphous forms of Boron and Aluminium form B2O3 and Al2O3 and in reaction with dinitrogen they form Nitrides.
2E (s) + 3O2 ⎯⎯→ 2E2O3 (s)
2E (s) + N2 (g) ⎯⎯→ 2EN (s)
[Where E = Element]
Nature of oxide:
Boron trioxide = acidic
Aluminium and gallium oxide = amphoteric
Indium and thallium oxide = basic
Reactivity towards acids and alkalis
- Boron doesn’t react with acids and alkalis at moderate temperatures.
- Aluminium is amphoteric, hence dissolves in mineral acids and liberates hydrogen.
2Al(s) + 6HCl (aq) → 2Al3+ (aq) + 6Cl– (aq) + 3H2 (g)
- Aluminium also reacts with alkali to form Sodium tetrahydroxoaluminate(III)
and liberate hydrogen gas.
2Al (s) + 2NaOH(aq) + 6H2O(l) → 2 Na+ [Al(OH)4] – (aq) + 3H2(g)
- Concentrated HNO3 renders aluminium and gallium passive due to the formation of a protective layer of oxide on its surface.
Reactivity towards halogens
Group 13 elements form trihalides on reaction with halogens (except TlI3).
2E(s) + 3 X2 (g) → 2EX3 (s)
[where X = F, Cl, Br, I]
Some trends and anomalous behavior of group 13 elements:
- Tri-chlorides, Bromides, and iodides of group 13 elements hydrolyse in water due to covalent nature. Tetrahedral [M(OH)4]‑ and octahedral [M(H2O)6]3+ compounds exist in an aqueous medium (except Boron).
- Monomeric trihalides are strong Lewis acids.
Boron trifluoride reacts with Lewis bases like NH3.
Borax
It is a white crystalline solid with the formula Na2B4O7.10 H2O.
Borax contains the tetranuclear unit [B4O5(OH)4]2- so the correct formula becomes Na2[B4O5(OH)4]. 8H2O.
On dissolving in water, Borax gives NaOH and Orthoboric acid (H3BO3).
Borax loses water from crystallization on heating and swells up to form sodium metaborate. It turns into a transparent liquid that solidifies to form a borax bead on further heating.
Na2B4O7.10H2O ⎯⎯→ Na2B4O7 ⎯⎯→ 2NaBO2 + B2O3
On heating Borax with CoO, a blue-colored bead of Co(BO2)2 is formed.
Orthoboric acid
Orthoboric acid (H3BO3) is a white crystalline solid with soapy touch.
Orthoboric acid is prepared by acidifying an aqueous solution of Borax.
Na2B4O7 + 2HCl + 5H2O → 2NaCl + 4B(OH)3
It is highly soluble in hot water but sparingly soluble in water.
It is a weak monobasic acid acting as a Lewis acid by accepting electrons from hydroxyl ions.
B(OH)3 + 2HOH → [B(OH)4] – + H3O+
Orthoboric acid forms meta-boric acid (HBO2) on heating at 370 K. On further heating, it yields boric oxide (B2O3).
Diborane
Diborane is the simplest boron hydride prepared by treating boron trifluoride with LiAlH4 in diethyl ether.
4BF3 + 3 LiAlH4 → 2B2H6 + 3LiF + 3AlF3
In the laboratory, oxidation of Sodium borohydride with iodine leads to the formation of Diborane.
2NaBH4 + I2 → B2H6 + 2NaI + H2
In industries, it is prepared by the reaction of BF3 with sodium hydride at 450 K.
2BF3 + 6NaH →B2H6 + 6NaF
Diborane is a colourless toxic gas with a boiling point of 180 K.
It burns vigorously in the presence of air, thus catching fire and releasing a large amount of energy.
Uses of Boron compounds
Boron is a hard refractory solid with a high melting point, low density, and very little conductivity. Due to this, it has various uses:
Boron fiber prepares bullet-proof vests and light composite material for aircraft.
Metal borides made up of Boron-10 isotope is used in the nuclear industry to prepare protective shields and control rods, since it can absorb neutrons.
Borax and boric acid are used in industries to manufacture heat-resistant glasses (pyrex), glass wool, and fiber glass.
Borax is a constituent of medicinal soaps and a flux for soldering metals.
Borax is helpful to prepare heat, scratch, and stain-resistant glazed coating on earthenware’s.
An aqueous solution of Orthoboric acid can be used as a mild antiseptic.
Uses of Aluminium compounds
Aluminium is a bright silvery-white metal. It has high tensile strength, high electrical and thermal conductivity. Since it shows toxic nature, aluminium and its compounds no longer have domestic purposes. Due to its chemical and physical properties, it has many industrial applications.
Aluminium combines copper, manganese, magnesium, silicon, and zinc to manufacture alloys.
Alloys of Aluminium are used to prepare pipes, tubes, rods, wires, plates, and foils.
Alloys have applications in the packaging, construction, airplane, and transportation industry.
Conclusion
In group 13 elements, valence electron lies in the p subshell. The chemical and physical properties of the elements vary due to the three electrons in the valence shell. Some group 13 elements show non-metallic character (Boron), while some show metallic character. They can be acidic, basic, or amphoteric.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






