Group 15 includes nitrogen, phosphorus, arsenic, antimony, and bismuth. The metalloid property causes a change from non-metallic to metallic as we move along the group. Non-metals nitrogen and phosphorus are non-metals, metalloids arsenic, and antimony are metalloids, while bismuth is a typical metal. These elements have an ns2np3 valence shell electronic configuration. The s orbital is totally filled, whereas the p orbital is half-filled, making them naturally stable. The oxidation states that these elements determine their chemical characteristics.
Structures of Oxides and Oxoacids of Nitrogen and Phosphorus
What are Oxoacids?
Simply described, oxyacids are acids that contain oxygen. One of these elements is phosphorus, which can be found in various oxoacids. Examples include H3PO4, H3PO3, and various oxyacids.
In phosphorus oxoacids, the phosphorus atom is tetrahedrally surrounded by other atoms. In general, these acids have at least one P=O bond and one P–OH connection. Phosphorus oxoacids have P–P or P–H bonds in addition to the P=O and P–OH connections. In these conditions, the oxidation state of phosphorus is less than +5.
Higher and lower oxidation states are prevalent in these acids. When phosphoric acid and phosphine are heated together, they produce phosphoric acid and phosphine.
Phosphorus
Phosphorus is a chemical element with the symbol P and the atomic number 15. Phosphorus exists in two major forms: white phosphorus and red phosphorus; however, its great reactivity is never found as a free element on Earth.
Phosphorus Oxoacids
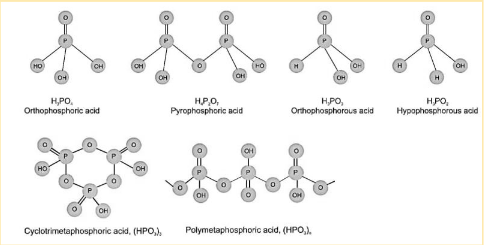
In oxoacids, the P-H bonds cannot be ionised to form H+ ions. On the other hand, H atoms linked to oxygen in P-OH form are ionisable. As a result, basicity can be defined as the property manifested by H atoms in contact with oxygen.
Phosphorus acid, H3PO3, is dibasic due to the two P-OH connections. Phosphoric acid, H3PO4, is tribasic because it has three P-OH linkages. The phosphorus oxoacids with P-H bonds have significant reducing characteristics. Hypophosphorous acid, for example, is an excellent reducing agent because it has two P-H bonds.
There are seven types of oxoacids of phosphorus.

Nitrogen
The chemical element nitrogen has the symbol N and the atomic number 7. Daniel Rutherford, a Scottish physician, was the first to discover and isolate it in 1772. Nitrogen is the first element of the 15th group and is the family of p-block.
Oxoacids of Nitrogen
Nitrogen forms five types of oxides.
- Dinitrogen monoxide or Nitrous oxide (N2O)
- Nitrogen monoxide or Nitric oxide (NO)
- Dinitrogen trioxide (N2O3)
- Nitrogen dioxide or Dinitrogen tetraoxide (N2O4)
- Dinitrogen pentoxide (N2O5)
Oxidation states of oxides of nitrogen are +1, +2, +3, +4, +5. The oxides of nitrogen are strongly acidic.
Nitrous Oxide
Nitrous oxide is a non-organic chemical made up of two nitrogen atoms and a single oxygen atom. Nitrous oxide is one of the nitrogen-related variable oxides. However, it does not fall under the category of NOx gases, which are responsible for air pollution. Joseph Priestley, a chemist, was the first to discover this gas.
Nitrous oxide is a gas that has a chemical name and a formula.
Nitrous oxide is known chemically as nitrogen (I) oxide or dinitrogen monoxide. Nitrous oxide has the chemical formula N2O, which stands for one atom of oxygen and two nitrogen atoms. Nitrous oxide gas has an oxidation state of +1, making it an unreactive gas.
Nitrous Oxide Structure
Nitrous oxide is an inorganic linear molecule. Within the molecule, there is a pi-bond that exhibits resonance. Nitrous oxide has a bond length of 119 picometers N-O bonds and 113 picometers N-N bonds in its overall structure.
Nitrous Oxide’s Characteristics
The following are the physical properties of nitrous oxide.
- Nitrous oxide is a gaseous substance
- In its natural state, it is colourless
- It has a neutral molecule
- It has a sweet flavour and a lovely aroma
- Nitrous oxide has a molecular weight of 44g/mol
- It has high water solubility
- In nature, nitrous oxide is non-combustible, and its vapour is much denser than air.
Conclusion
Nitrogen, phosphorus, arsenic, antimony, and bismuth make up Group 15. Like those in the previous groups, elements in this group have a regular gradation in physical and chemical properties. While nitrogen and phosphorus are non-metallic, arsenic and antimony are metalloids, and metallic properties predominate in bismuth. Nitrogen, the first element in the group, exhibits certain unusual characteristics. In oxoacids, the P-H bonds cannot be ionised to form H+ ions. On the other hand, H atoms linked to oxygen in P-OH form are ionisable. As a result, basicity can be defined as the property manifested by H atoms in contact with oxygen. Nitrogen is a group of 15 elements that can be broken down into three oxoacids. Hyponitrous acid, nitrous acid, and nitric acid are the three acids. NO2, a nitrogen and oxygen-based gaseous air pollutant, is a group of gases known as nitrogen oxides.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






