Any chemical reaction that changes the oxidation number of a molecule, atom, or ion by gaining or losing an electron is an oxidation-reduction reaction. Photosynthesis, respiration, combustion, and corrosion or rusting are all examples of redox reactions that are common and necessary for some of life’s most basic functions. Some species oxidise, or lose electrons, while others reduce or gain electrons during a redox reaction.
The chemical species from which the electron is removed is referred to as oxidised, whereas the chemical species to which the electron is added is referred to as reduced.
It is important to note that oxidation and reduction do not occur just between metals. Electrons can also travel between metals and nonmetals.
In redox reactions, the reduction agent transfers electrons to the oxidising agent. Thus, in the reaction, the reductant or reducing agent releases electrons and is oxidised, while the oxidant or oxidising agent gains electrons and is reduced. A redox pair is an oxidising and reducing agent pair involved in a specific reaction.
Oxidizers or Oxidising Agent
Substances that can oxidise other substances (cause them to lose electrons) are referred to as oxidative or oxidising agents, oxidants, or oxidizers. The oxidising agent is also known as an electron acceptor because it “accepts” electrons. The most basic oxidizer is oxygen. Oxidants are typically chemical substances with high oxidation states (H2O2, MnO−4, CrO3, Cr2O2−7, OsO4) or highly electronegative elements (O2, F2, Cl2, Br2) that can add extra electrons by oxidising another substance.
Reducers or Reducing Agent
Substances that can reduce other substances (cause them to gain electrons) are referred to as reductive or reducing agents, reductants, or reducers. The reducing agent is also known as an electron donor because it donates electrons. In chemistry, there are numerous types of reducants. Lithium, sodium, magnesium, iron, zinc, and aluminium are all good reducing agents. These metals are relatively easy to donate or give away electrons.
Oxidation numbers
It describes the degree to which an atom in a chemical compound has been oxidised. In theory, the oxidation state can be positive, negative, or zero.
The atoms in a reaction can be assigned oxidation numbers using the following guidelines:
- An individual atom has an oxidation state of 0.
- The total oxidation state of all atoms in a neutral species is 0, while it is equal to the ion charge in an ion.
- The oxidation state of metals in Group 1 is +1, while metals in Group 2 have an oxidation state of +2.
- In most compounds, fluorine has an oxidation number of -1, oxygen has an oxidation number of -2, and hydrogen has an oxidation state of +1.
- In binary metal compounds, Group 17 elements have an oxidation state of -1, Group 16 elements have a state of -2, and Group 15 have a state of -3.
- The sum of the oxidation numbers for all atoms in a neutral compound is zero, whereas the sum for all atoms in a polyatomic ion equals the ion’s charge.
Types Of Redox Reaction
- Combination Reaction: It happens when the reaction of two reactants results in the formation of a product. When we burn magnesium ribbon (or magnesium), we get grey-black ash of magnesium oxide, an example of a combination reaction.
Sample equation – A + B → AB
Equation example – 4Fe + 3O2→2Fe2O
- Decomposition Reaction: It happens when a compound decomposes to produce two or more products. For instance, consider water electrolysis. Water is electrolyzed to produce hydrogen and oxygen, which have completely different properties than water. General equation – AB→ A + B
Equation example – 2H2O → 2H2 + O2
- Displacement Reaction: In these reactions, more reactive metal displaces less reactive metal from its salt. They can take place as single or double replacement reactions.
General equation – A + BC → AB + CA (single displacement)
Equation example – 2K + MgCl2 → 2KCl + Mg
- Disproportionation Reactions: A reaction in which the same atom is oxidised and reduced simultaneously. A typical example of this type of reaction is the decomposition of hydrogen peroxide.
General equation – 2A → A’ + A”
Equation example – 2 H2O2(aq) → 2 H2O(l) + O2(g)
Examples of Redox Reaction
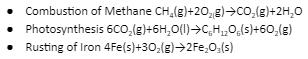
Conclusion
Redox reactions, also known as oxidation–reduction reactions, involve the transfer of electrons from one species to another. The species that loses electrons is referred to as oxidised, whereas the species that gains electrons is referred to as reduced.
We can identify redox reactions by assigning oxidation numbers to atoms in molecules and assuming that all bonds to the atoms are ionic. An increase in oxidation number during a reaction indicates oxidation, while a decrease indicates reduction.
The ubiquity of redox reactions occurs across a wide range of diverse chemical reactions encountered in essential life functions, from chemical reactions experienced in everyday life to industrial processes. Redox reactions can be applied in both real-life situations and industrial fields.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






