Introduction: What are intermolecular forces?
The forces of attraction and repulsion between interacting particles are known as intermolecular forces (atoms and molecules). The electrostatic forces that occur between two oppositely charged ions and the forces that keep atoms of a molecule together, i.e. covalent bonds, are not included in this term.Van der Waals forces are attractive intermolecular forces named after Dutch physicist Johannes van der Waals (1837-1923), who used these forces to explain how real gases differ from ideal behaviour. Dispersion forces, also known as London forces, dipole-dipole forces, and dipole-induced dipole forces are all examples of van der Waals forces. Hydrogen bonding is a sort of dipole-dipole interaction that is exceptionally strong.
Because only a few elements may participate in hydrogen bond production, it is classified as a distinct category. It is vital to clarify that the attractive forces between an ion and a dipole are referred to as ion-dipole forces and are not van der Waals forces.
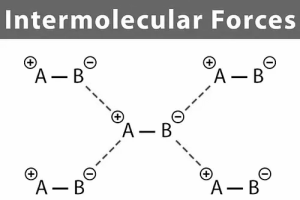
Different types of Intermolecular forces:
Dispersion forces or London forces:
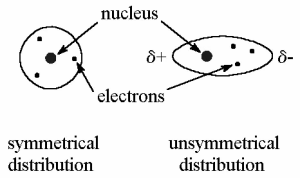
Because of the mobility of electrons, dispersion forces or London forces produce brief positively and negatively charged zones. Because the electrically charged cloud of atoms and nonpolar molecules is symmetrically distributed, they lack a dipole moment. However, atoms and molecules may exhibit dipole characteristics for a short period of time. These are the weakest forces, and they can only go a short distance.
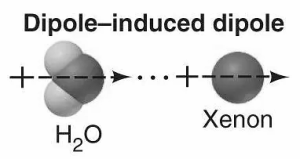
Dipole-induced dipole interaction:
The presence of polar molecules changes the non-polar molecules into induced molecules and results in the dipole-induced dipole interaction. The interaction takes place between the molecules without dipoles and the polar moles with dipoles. The other molecule becomes a dipole as the permanent dipole of the polar molecules makes the electrically neutral molecule’s dipole by damaging its electron cloud.
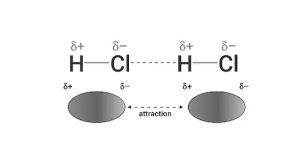
Dipole-dipole interaction:
Polar compounds have dipole-dipole interactions. Permanent dipoles in polar molecules result from a difference in the electronegativity of the atom caused by covalent connections. When two molecules interact, the positive component of one molecule is attracted to the negative part of another molecule. As a result, the dipole-dipole interaction is the force caused by the attraction.
Ion induced dipole interaction:
Placing an ion near a nonpolar molecule causes it to become polar. The non-polar molecules behave like induced dipoles when charged. Ion-induced interaction is the force formed between an induced dipole and an ion. The strength of intermolecular forces is determined by the tendency of nonpolar molecules to become charged.
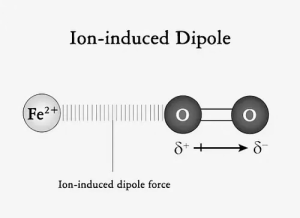
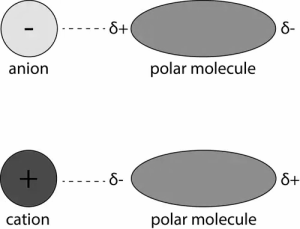
Ion dipole interaction:
The ion-dipole interaction creates a connection between polar molecules and ions. The intensity of the interaction is determined by the following factors:
- An ion’s charge and size.
- The dipole moment’s percentage.
- The polar molecule’s magnitude.
Intermolecular forces can be seen in sodium chloride molecules dissolved in water. Negative ions (Cl-) exist in chlorine, while positive ions (Na+) exist in sodium. Water with polar molecules is attracted to ions with opposing charges.
Things to Remember About Intermolecular Forces:
- The attraction and repulsion forces that emerge between the molecules/atoms of a substance are known as intermolecular forces.
- Intermolecular forces, or IMF, are the electrostatic forces that exist between molecules and atoms.
- The intermolecular forces are directly proportional to the boiling point of various substances.
- The dispersion forces, often known as London forces, are the weakest.
- Similar to dipole-dipole interactions, ion-dipole interactions occur between ions and polar molecules.
What Effect Do Intermolecular Forces Have on Boiling Point?
The electrostatic intermolecular forces are substantially less than the chemical forces. They can be found in all states of matter and play a vital role in determining a variety of structural and physical properties.
The boiling point of a substance can be used to estimate the intensity of intermolecular forces operating among the molecules of that substance. The magnitude of intermolecular forces increases as the boiling point rises. Similarly, as the strength of intermolecular interactions increases, so does the melting point of a substance.
Conclusion:
Intermolecular forces exist between matter particles. Pure electrostatic forces exist between two oppositely charged ions, but these forces are different. These forces do not include the covalent bonds that hold atoms in a covalent molecule together. The state of matter is determined by the competition between thermal energy and intermolecular interactions.The energy of constituent particles and the sort of interaction between them determine “bulk” qualities of matter such as gas behaviour, solids and liquids characteristics, and state change. The chemical properties of a substance do not change as a result of its physical condition, but its reactivity does.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






