Overview
Hydrogen peroxide is a frequently used chemical compound used as a cleanser by numerous surgeons and homemakers. This substance is volatile and is denser than water in its pure form.
Combined with 2 molecules of oxygen to form hydrogen peroxide, the compound’s chemical formula is H2O2. The substance is the most straightforward peroxide available (oxygen-oxygen single bond), having basic uses as a bleaching agent, oxidizer, and antiseptic.
The substance, also known as high-test peroxide, is a pale blue and clear liquid also used as a propellant in rocket engines in concentrated form.
Hydrogen Peroxide Structure
The structure of hydrogen peroxide is non-planar. There are 2 planes, and each plane has one O-H bond. The angle between the planes is 90.2o. Plus, the bond length is 145.8 pm, and length of the O-H bond is 98.8pm.
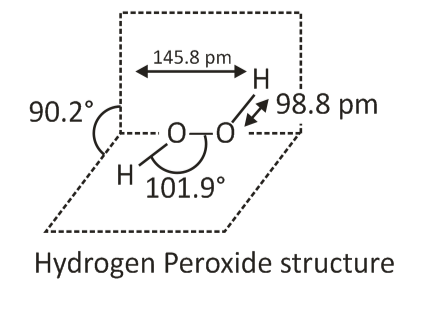
It proves the valence shell electron pair repulsion theory correct as each oxygen atom contains 2 lone pairs of electrons. The unbonded electrons of oxygen are repelled by hydrogen atoms leading to forming the bent molecular shape.
Preparation of Hydrogen Peroxide
Hydrogen Peroxide can be prepared by 3 methods. They are described in detail here.
Method 1: Laboratory Preparation of Hydrogen Peroxide
Hydrogen peroxide can be prepared from barium peroxide. Removing excess water by the process of evaporation under reduced pressure, while acidifying barium peroxide, is one of the ways to obtain a chemical compound named hydrogen peroxide. The statement can be justified with the help of a reaction mentioned below:
BaO2.8H2O(s) + H2SO4(aq) → BaSO4(s) + H2O2(aq) + 8H2O(l)
The only limitation of preparing H2O2 through barium peroxide is that the solution contains many Ba2+ ions that catalyze the decomposition of hydrogen peroxide. This does not let the substance to be stored for a very long duration and makes it unstable and excessively explosive. As a result, it is preserved in a water solution when prepared from the laboratory method.
Method 2: Commercial Preparation of Hydrogen Peroxide
Hydrogen peroxide can also be produced through the auto-oxidation method.
Method 3: Industrial Preparation of Hydrogen Peroxide
Merck’s preparation method: We get the mixture of hydrogen peroxide and sodium sulphate by adding sodium peroxide to a cold solution of 20% sulfuric acid. At the same time, cooling the crystals of sodium sulphate and separating the solution results in obtaining 30% hydrogen peroxide.
Na2O2 + H2SO4 → Na2SO4 + H2O2
Many industries prepare the substance by electrolysis of 30% ice-cold sulphuric acid. We get the peroxodisulphate when the acidified sulphate solution is electrolyzed at an extremely high current density. When the peroxodisulphate is hydrolyzed, hydrogen peroxide is obtained. The following reaction will help you understand how it is done:
2HSO4-(aq) [Electrolysis] → HO3SOOSO3H (aq)
HO3SOOSO3H(aq) [Hydrolysis] → 2HSO4- (aq) + 2H+(aq) + H2O2(aq)
Properties of Hydrogen Peroxide
Physical Properties
- Hydrogen peroxide is miscible with water, alcohol, and all forms of hydrates.
- It is bitter in taste.
- It is a colourless (pale blue) and odourless liquid.
- Pure hydrogen peroxide is a thick syrupy liquid.
- The substance boils at 423 K (extrapolated) and has a melting point of 272.4 K.
Chemical Properties
- When mixed with acidic and basic medium, the solution acts as an oxidizing and reducing agent.
- Because of its high instability, it decomposes well into water and oxygen.
Reaction: 2H2O2鈥(aq)→2H2O(l)+O2(g)
- In an acidic medium, hydrogen peroxide oxidizes acidified ferrous sulphate to ferric sulphate.
Reaction: 2FeSO4+H2SO4+H2O2→Fe2(SO4)3鈥+H2O
- In a neutral solution, hydrogen peroxide oxidizes lead sulphide to lead sulphate.
Reaction: PbS(s)+4H2O2(aq)→PbSO4(s)+4H2O(l)
- Oxidizes nitrates to nitrites.
Reaction: NaNO2 + H2O2 → NaNO3 + H2O
- Oxidizes sulphates to sulphates.
Reaction: Na2SO3 + H2O2 → Na2SO4 + H2O
- Oxidizes black Pbs to white PbSO4
Reaction: Pbs + 4H2O2 → PbSO4 + 4H2O
- The chemical compound can take up an oxygen atom to provide oxygen gas and water, as it acts as a reducing agent in the presence of other oxidizing agents.
Reaction: Ag2O + H2O2→ 2Ag + H2O + O2
Uses of hydrogen peroxide
Mentioned below are the multiple uses of hydrogen peroxide:
- Hydrogen peroxide is very effective on wounds as it acts as an antiseptic.
- It acts as a bleaching agent in the textile and paper industries.
- The substance is used in wastewater treatment, especially inorganic chemicals like sodium perborate or other impurities.
- The compound can be used to lighten your hair as it has the properties to dye your hair color.
- It can be used to sanitize your toothbrush, makeup brush, and whiten your teeth.
- In case of any mishaps with your pets, doctors will advise you to use hydrogen peroxide to induce vomiting for poisoned pets.
- The solution is also used to detect titanium or chromium in the laboratory.
- The manufacturing of sodium percarbonate and sodium perborate becomes easy with hydrogen peroxide. These produced substances are further used for major industrial applications.
- The chemical is also used to produce organic peroxides with dibenzoyl peroxide.
- H2O2 is also used for several household purposes. It can shine mirrors and glass surfaces, deep clean your toilet, remove stains on clothes, scour cookware, wash veggies, and extend their shelf life, scrub your sink, and disinfect counters or cutting boards.
- It can produce alternative medicine and may cure conditions including AIDS, cancer, influenza, and emphysema.
- It also facilitates fish aeration and hydroponic farmers to get stupendous results by mixing a small amount of hydrogen peroxide in watering solutions, leading to better production output.
Conclusion
Hydrogen peroxide is a frequently used chemical compound by surgeons and homemakers. It is a volatile substance and is denser than water in its pure form. Due to its unique chemical and physical properties, hydrogen peroxide is used widely in industrial, commercial, and laboratory applications.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




