Introduction
Whenever various chemical reactions occur it produces heat, light, or sound as a byproduct. This is an exothermic process. Exothermic reactions can occur spontaneously and increase the system’s unpredictability or entropy (ΔS > 0). They are distinguished by a drop in enthalpy (ΔH < 0) and a negative heat flow (heat is lost to the environment). Exothermic reactions in the lab generate heat and might be explosive.
Other chemical reactions need the absorption of energy in order to continue. They’re called Endothermic reactions. Endothermic reactions cannot occur on their own. Work is required in order to get these reactions to occur. When endothermic processes consume energy, the temperature drops during the reaction.
Positive heat flow (into the reaction) and a rise in enthalpy (+ΔH) define endothermic reactions.
What exactly is a chemical reaction?
Chemical reactions are the processes by which a material or substances change to form new compounds with new attributes.
When calcium carbonate is heated, for example, calcium oxide (lime) and carbon dioxide are produced. Similarly, the transformation of calcium carbonate into calcium oxide and carbon dioxide is a chemical reaction because calcium carbonate transforms into new molecules, calcium oxide and carbon dioxide.
In a chemical reaction, just atoms are rearranged; no new atoms are produced.
The chemicals that participate in a chemical reaction are referred to as reactants, while the new compounds created are referred to as products. The characteristics of the products created are completely different from those of the reactants.
What Is the Definition of an Endothermic Reaction?
Energy is involved in all chemical processes. When reactants break bonds, energy is released, and when products make new bonds, energy is released. Exothermic reactions are those in which more energy is released when new bonds form in the products than is required to break bonds in the reactants. Endothermic reactions are the polar opposite of exothermic processes. It takes more energy in order to break bonds in the reactants than is released when the new bonds are formed in the products in an endothermic reaction.
Energy Change in Endothermic Reaction
The term endothermic literally means “heating up.” An endothermic process requires a steady input of energy, which is commonly in the form of heat. This is seen in the diagram below. Because not enough energy is released when the products form to break additional bonds in the reactants, energy must be continually provided. An endothermic reaction has the following general equation:
Reactants + Energy → Products
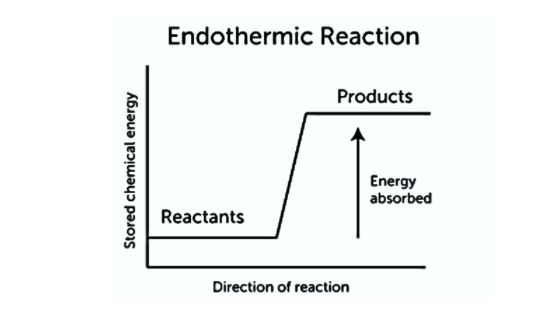
The temperature of the products in endothermic reactions is often lower than the temperature of the reactants. The temperature change might be significant enough to cause liquids to freeze.
What Exactly Is An Exothermic Reaction?
Energy is involved in all chemical processes. When reactants break bonds, energy is released, and when products make new bonds, energy is released. Endothermic reactions are those in which less energy is produced when new bonds form in the products than is required to break bonds in the reactants. Exothermic reactions are the polar opposite of endothermic processes. In an exothermic reaction, the energy required to break bonds in the reactants is less than the energy released when new bonds form in the products.
Energy Change in Exothermic Reaction
The term exothermic implies “heating up.” As an exothermic process progresses, energy, frequently in the form of heat, is released. This is seen in the diagram below. An exothermic reaction has the following general equation:
Reactants → Products + Energy
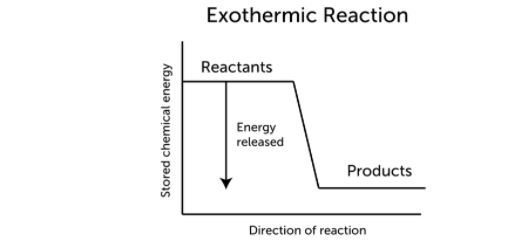
When the energy created in an exothermic reaction is released as heat, the temperature rises. As a result, the products will most likely be hotter than the reactants.
How to know when a Reaction in Exothermic or Endothermic
There are two ways to tell the difference between exothermic and endothermic processes.
- Keep track of temperature changes.
The temperature of the reaction mixture rises as energy is released in an exothermic process. The temperature drops as energy is absorbed in an endothermic process. Placing a thermometer in the reaction mixture allows you to observe temperature changes.
- Determine the reaction enthalpy (ΔH)
The enthalpy change (H) or heat of reaction, which compares the energy of the reactants with the energy of the products, can be used to identify the net energy output or input of chemical processes.
Enthalpy is a unit of measurement for internal energy. So, when you subtract the enthalpy of the products from the enthalpy of the reactants, you get the enthalpy change (ΔH), which can be expressed mathematically as:
H = energy produced in product bond formation – energy used in reactant bond breaking
Conclusion
Exothermic reactions are those in which more energy is released when new bonds form in the products than is required to break bonds in the reactants. As It takes more energy in order to break bonds in the reactants than is released when the new bonds is form in the products in an endothermic reaction. An endothermic process requires a steady input of energy, which is commonly in the form of heat. Endothermic reactions are those in which less energy is produced when new bonds form in the products than is required to break bonds in the reactants. Exothermic reactions are the polar opposite of endothermic processes. In an exothermic reaction, the energy required to break bonds in the reactants is less than the energy released when new bonds form in the products.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






