In the shell atomic model, an atom’s electrical configuration can be described by listing the number of electrons in each shell, starting with the first. The 11 electrons in sodium (atomic number 11) are distributed as follows: the K and L shells are totally filled with 2 and 8 electrons, respectively, while the M shell is only half filled with one electron.
In the quantum-mechanical concept, an atom’s electronic configuration is expressed by listing the occupied orbitals in order of filling, with the number of electrons in each orbital indicated by a superscript. The electrical configuration of sodium in this notation is 1s2 2s2 2p6 3s1, which is dispersed in the orbitals as 2-8-1. The electrons in excess of the noble gas configuration immediately preceding the atom in the periodic table are frequently listed using a shorthand manner. Because sodium (chemical symbol Ne, atomic number 10) has one more 3s electron than the noble gas neon (chemical symbol Ne, atomic number 10), its shorthand notation is [Ne]3s1.
Electron Orbitals Rules for Filling
We must follow three rules when assigning electrons to orbitals:
- Aufbau Principle
- Pauli-Exclusion Principle
- Hund’s Rule
Aufbau Principle
The ground state electron configuration in atoms/ions with two or more electrons (1) minimizes the total energy of the electrons, (2) follows the Pauli Exclusion Principle, (3) follows the Hund’s rule of maximum multiplicity, and (4) takes the exchange interaction into account.
In an atom’s or ion’s ground state, electrons occupy the lowest-energy subshells first, then higher-energy subshells. For example, the 1s subshell is occupied before the 2s subshell. An atom’s or ion’s electrons form the most stable electron configuration feasible in this fashion. The phosphorus atom, for example, has the configuration 1s2 2s2 2p6 3s2 3p3, indicating that the 1s subshell possesses two electrons, and so on.
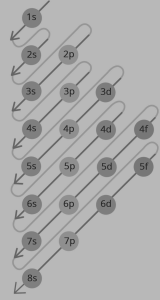
Figure: 1 Aufbau Principle
Pauli-Exclusion Principle
According to Pauli’s Exclusion Principle, no two electrons in the same atom may have the same values for all four quantum numbers. To put it another way, (1) only two electrons can occupy the same orbital, and (2) the spins of two electrons in the same orbital must be opposing.
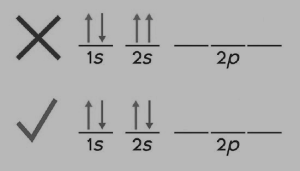
Figure: 2 Pauli’s Exclusion Principle
Hund’s Rule
The lowest energy atomic state is the one that maximizes the total spin quantum number for the electrons in the open subshell, according to Hund’s first rule. Before double occupation, the orbitals of the subshell are each occupied singly with electrons of parallel spin.

Figure: 3 Hund’s rule
Electronic Configuration Writing
Starting at the top of the periodic table and working your way down the rows from left to right, write the row number, block letter, and the number of squares in each block’s section until you reach the desired element. Write 1s2 2s2 2p6 3s2 3p3 for the electron configuration of phosphorus (P), which is in the third row, p-block, third element in that block. Check your work by adding the electron numbers to see if they equal the element’s atomic number; for instance, 2+2+6+2+3=15, which is the atomic number of phosphorus.
Electron configurations are written using three methods:
- spdf notation
- noble gas notation
- orbital diagrams
Shells
An electron shell is the region of an atom that surrounds the atomic nucleus on the outside. It’s a collection of atomic orbitals with the same n-th main quantum number. One or more electron subshells, or sublevels, exist in electron shells.
Subshells
A subdivision of electron shells separated by electron orbitals is called a subshell. In an electron configuration, subshells are labelled s, p, d, and f.
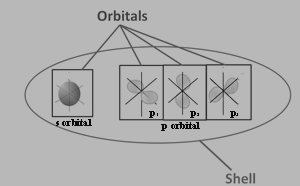
Orbitals
The number of electrons, as well as the orbitals. The number and letter indicate the energy level and orbital, respectively, and the superscript number indicates how many electrons are in that orbital.
Figure: 4
Electronic Configuration Applications
- The interpretation of atomic spectra is a key application of electron configurations. In this situation, one or more term symbols that describe the various energy levels available to an atom must be added to the electron configuration. Although not all energy levels are observed in practice, term symbols are used to represent them. Can be derived for any electron configuration, not simply the ground-state configuration shown in tables. The ground-state electron configurations of the elements were experimentally determined using atomic spectra analysis.
- Any multi-electron system requires a vast number of electronic configurations to accurately characterise it, and no energy can be assigned to a single configuration. However, because the electronic wave function is typically dominated by a small number of configurations, the concept of electronic configuration is still important in multi-electron systems.
Conclusion
The distribution of electrons in an element’s atomic orbitals is described by its electron configuration.
The electron configuration is used to represent an atom that has discharged into a cation or anion by compensating for the loss or gain of electrons in succeeding orbitals. In both inorganic and organic chemistry, the most common application of electron configurations is the reduction of chemical characteristics. In effect, electron configurations, in combination with a simplified version of molecular orbital theory, have evolved into the modern counterpart of the valence notion, which describes the number and type of chemical bonds that an atom is likely to make.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out