The electromotive force of a galvanic cell constructed from a standard reference electrode and another electrode to be characterized is known as electrode potential in electrochemistry. The standard hydrogen electrode is used as the reference electrode by convention (SHE). It is defined as having a zero-volt potential. The potential difference between the electrodes, which is measured in volts (v), is determined by the compounds that make up the electrodes. The total potential of any electric cell is the sum of the potentials produced by the reactions at the two electrodes –
EMF oxidation + EMF reduction = EMF cell
Electrode Theory
The entire theory of electrode conduct, including single potential determinations, overvoltage, transfer resistance, valve action, and passivity, has been studied. Normal electrode potentials are periodic functions of element atomic numbers, and inert gas electrode potentials are most likely zero.
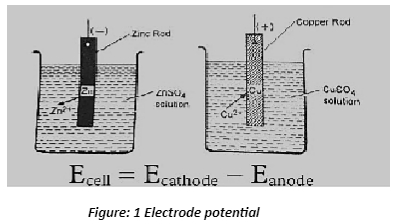
The several forms of electrode potential
Oxidation potential – occurs when the electrode is negatively charged in relation to the solution.
Reduction potential – When the electrode is positively charged in relation to the solution, it has a reduction potential.
Example of electrode potential
When a zinc plate is immersed in a solution containing Zn2+ ions, it gets negatively charged in relation to the solution, resulting in a potential difference between the zinc plate and the solution. The electrode potential of zinc is the difference in potential.
Redox Reactions
Redox is a term for reduction-oxidation, which means that a redox reaction has both a reduction and an oxidation process going on at the same time. It can also be used as a shorthand for the oxidation-reduction reaction. Let’s look at these two elements separately before returning to how they
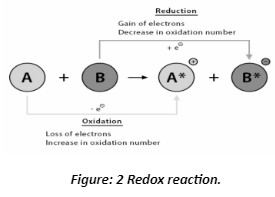
Reduction – During a chemical process, an atom gains one or more electrons, which is known as reduction. Its oxidation number drops as a result. Because an electron carries a negative charge, an atom gains a negative charge when it gains an electron, lowering the oxidation number. For example, an atom could transition from X2+ to X1+ or X0 to X1- . As the oxidation number decreases, this can help us recall what reduction is.
Cu2+ (aq) → Cu (s)
F2 (g) → 2F– (g)
Oxidation – When an atom loses one or more electrons during a chemical reaction, the oxidation number of the atom increases. This is due to the atom losing its electron’s negative charge, which is equivalent to receiving a positive charge, therefore increasing the oxidation number. For example, an atom could transition from X1- to X0 or X0 to X1+. Because the first redox processes detected were those involving oxygen, the term “oxidation” is employed. However, because the oxidation number increases/becomes more positive, we can think of it as oxidation if that helps.
Types of Redox reaction
The various types of redox reactions are listed below,
- Decomposition Reaction
- Combination Reaction
- Displacement Reaction
- Disproportionation Reaction
Advantage: The importance of redox reactions in biological reactions cannot be overstated. The electron transfer system in cells, for example, and glucose oxidation are examples of redox reactions in the human body. Redox reactions yield a variety of chemical compounds that are valuable in industrial activities.
Standard electrode potential
The equilibrium potential is measured by the standard electrode potential. The difference in potential between the electrolyte and the electrode is the electrode’s potential. The electrode potential is referred to as the standard electrode potential when the concentrations of all species in a semi-cell are equal.
In an electrochemical cell, the standard electrode potential arises under normal conditions, such as
- 298K temperature,
- 1atm pressure,
- 1M concentration
The standard electrode potential of a cell is denoted by the symbol ‘EO cell.
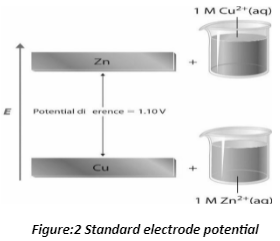
In the Zn/Cu system, there is a potential energy difference. The potential energy of a system made up of metallic Zn and aqueous Cu2+ ions is higher than that of a system made up of metallic Cu and aqueous Zn2+ ions. The valence electrons of metallic Zn have a higher energy than the valence electrons of metallic Cu, which accounts for a large portion of the potential energy difference. When electrons are moved from Zn to Cu2+ to create Cu and Zn2, energy is released because the Zn(s) + Cu2+ (aq) system has 1.10 V more energy than the Cu(s) + Zn2+ (aq) system.
Standard Electrode Potential’s Importance
Redox processes, which are made up of two parts, underpin all electrochemical cells.
The oxidation half-reaction, which involves the loss of electrons, occurs at the anode.
A reduction process happens at the cathode, giving in an electron gain. Electrons pass from the anode to the cathode as a result.
The electric potential between the anode and the cathode is caused by the difference in the individual potentials of each electrode.
As a reference electrode, a standard hydrogen electrode (SHE) is used. SHE’s electrode potential is 0 volts.
By attaching an electrode to the SHE and taking a reading on the cell potential of the resulting galvanic cell, the standard electrode potential of the electrode can be established.
Excellent oxidising agents have large standard reduction potentials, whereas good reducing agents have low standard reduction potentials.
Conclusion
The electromotive force of a galvanic cell constructed from a standard reference electrode and another electrode to be characterised is known as electrode potential in electrochemistry. The standard hydrogen electrode is used as the reference electrode by convention (SHE). It is defined as having a zero-volt potential. The electromotive force of a cell made up of two electrodes is known as the electrode potential in electrochemistry. The letter E stands for it. It is impossible to directly quantify the absolute value of a single electrode potential.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






