Adsorption is a process that involves the accumulation of material in molecular form in increasing concentrations on a surface as a result of the interaction of the substance with the surface. Consider the gases hydrogen, nitrogen, and oxygen. These gases adsorb on activated charcoal, which is a type of carbon black. Meanwhile, it is important to remember that adsorption is distinct from absorption. The mechanisms involved in the two processes are completely different.
There are two components necessary for the adsorption process, which are as follows:
It is a material that deposits on the surface of another substance and is known as an adsorbate. For example, the gases H2, N2, and O2.
Adsorbent: The surface of a substance on which an adsorbate binds. For example, charcoal, silica gel, and alumina are all-natural materials.
Adsorption can be classified into two types based on the interaction forces that exist between the adsorbate and the adsorbent.
Physical adsorption: Also known as physisorption, this form of adsorption is characterised by the presence of a physical barrier. Due to weak Van der Waals forces between the adsorbate and the adsorbent, this occurs.
For example, the gases H2 and N2 adsorb on coconut charcoal when exposed to heat.
Chemistry Adsorption: Also known as chemisorption, this type of adsorption involves the use of chemicals to adhere to a surface. Adsorbate and adsorbent are bonded together by strong chemical forces of the bonding type, and this causes adsorption. The creation of iron nitride on the surface of iron when it is heated at 623 K in N2 gas is a good illustration of what we mean.
It is a spontaneous exothermic reaction that takes place when gas is absorbed onto a solid. A unit mass of gas is adsorbed on a surface, and the quantity of heat liberated as a result is referred to as the heat of adsorption.
Physical adsorption
Most particles in contact with a solid or liquid surface will adhere to it through physical adsorption, also known as physisorption, and this is the most common type of adsorption. A good example is the condensation of water molecules on the surface of a drinking glass. An extremely cold glass will accumulate hundreds or thousands of layers of water molecules because each new layer of water molecules can adhere to the layer that came before it. incoming water molecules do not require a “spot” on the glass surface to adhere to the surface, as previously stated.
The top of this glass is exposed to the elements and therefore slightly warmer. Water molecules (which are not drawn to scale) can stick to the top part of the glass as a result of this effect on the water molecules. Warming the glass again will allow the condensation to drip off because the physisorption will become weaker as the atoms in the glass move about at a higher temperature as the glass is warmed again. This causes water molecules to receive an additional burst of energy on occasion, just enough to liberate them from their sticky neighbours and melt away from the surface of a glass. In other words, the molecules can desorb from the surface of the water. Adsorption and desorption are opposed.
Properties of Physical Adsorption
It is reversible and has a relatively low heat of absorption (about 5kcal/mole).
Adsorption is more pronounced when the temperature of the absorbate is lower than the boiling point.
This adsorption is always multilayer in nature and is virtually completely non-specific.
Physical adsorption is more a function of the absorbate than the other way around.
There is no significant specific heat of activation associated with this absorption.
Chemical adsorption
In contrast to physisorption, chemical adsorption, also known as chemisorption, is characterised by chemical interactions that can range from very weak to at least 100 times greater than those found in physisorption. A certain amount of chemistry is involved in chemical adsorption (as implied by the name). In other words, the creation of chemical bonds is required. A particular surface can chemisorb some molecules, whereas others are not chemisorbable.
Because chemisorption is a chemical process, it is frequently accompanied by a significant amount of activation energy. When molecules are heated to a specific temperature, they have varying quantities of energy as they tumble around our cosmos. “Temperature” refers to the average kinetic energy, or energy of motion of a sample of molecules, which is what we mean when we say “temperature.” Chemisorption can only occur in samples containing molecules with at least the activation energy, much as how only persons standing at or over a specific height are permitted to ride dangerous roller coasters at an amusement park.
Properties of Chemical Adsorption
It is estimated that the heat of chemical absorption is 20 to 100 times higher than the heat of physical adsorption (approximately 20 to 100 kcal/mole). The bond is strong, and the state of equilibrium is achieved gradually.
Chemical Adsorption is more pronounced usually at a high temperature.
In nature, this adsorption occurs mostly on monolayers and is very particular in its behaviour.
Adsorption by chemical means has a typical function for both adsorbate and adsorbent. This absorption has a significant amount of heat of activation.
Isotherm of Adsorption
Isotherms are commonly used to characterise adsorption processes. It is because temperature plays a significant part or has a significant impact on the entire process, as explained above. Furthermore, numerous isotherm models are employed to characterise the adsorption process, each of which has its own set of limitations. The Freundlich Theory is one of them.
Adsorption occurs when the adsorbate forms a monomolecular layer on the surface of the adsorbent, and the Freundlich adsorption isotherm is obeyed by the adsorption.
− x/m = k.
Pressure is represented by the symbol P1/n (n > 1) x, where x is the amount of gas adsorbed on one gramme of adsorbent, K and N denote the adsorption constants, and ‘p’ denotes the pressure with n always greater than one.
A significant disadvantage of the Freundlich adsorption isotherm is that it fails at high pressure. The multi-layered adsorption process could not be explained by this model.
Langmuir Theory
Langmuir proposed the hypothesis of adsorption of a gas on the surface of a solid in 1916, claiming that the solid’s surface was composed of elementary sites, each of which could adsorb a single gas. Because all adsorption sites are considered equal, it is presumed that a gas molecule’s ability to become bonded to any one site is independent of whether or not the adjacent sites are occupied. Additionally, it is believed that there is a dynamic equilibrium between the adsorbed and non-adsorbed gas molecules in the system.
The Langmuir adsorption isotherm can be used to derive the following principles:
- The gas adsorbed has the best behaviour in the vapour phase.
- Only monolayer adsorption occurs in this case.
- The surface of the solid is smooth and homogeneous in appearance.
- There is no lateral interaction force between the adsorbate molecule and the other adsorbate molecule.
- The adsorbed gas molecules have a specific location.
BET theory (after Brunauer, Emmett, and Teller)
Brunauer, Emmett, and Teller proposed the BET theory in 1938, and it has been in use since. Physisorption results in the production of multilayer adsorption, which is explained by this hypothesis. This theory also discusses the homogeneity of adsorption sites on solid surfaces, which is another important consideration. It is presumptively true that when adsorption occurs at one location, it will have no effect on adsorption at nearby sites.
Conclusion
As a key activity in ecology, it regulates the exchanges between the geosphere, water column, and atmosphere; it is responsible for the transfer of compounds in ecosystems, and it is the initiator of many other essential processes such as ionic exchange and enzymatic reactions. In addition to being one of the oldest techniques, adsorption is also one of the most important factors in making any industry today vital in its applications and use. It is utilised in a variety of industries, including petroleum, dyes, and food businesses such as oils, dairy, and a variety of other industries that are too numerous to list. It is sufficient to remind out that there is virtually no extant industry in the civil and military sectors that is not influenced by adsorption procedures. Water treatment and purification, particularly those resulting from various industrial processes and sewage water, are two of the most common applications of the adsorption process. These processes remove any traces of polluting substances that are extremely toxic to the environment and society, as well as the colour, taste, and odour of water that has been contaminated by pollution, among other things.
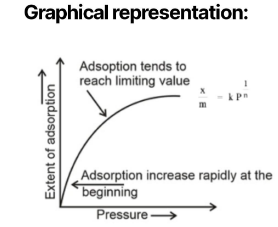
at low pressure 1/n = 1
.. x/m ∝ P
Consequently, at low pressure, the degree of adsorption is precisely proportional to the pressure, and so, at low pressure, the graph is a straight line (see Figure 1).
At medium pressure
At medium pressure 0 <1/n < 1
i. e, x/m ∝ p1/n
In other words, at medium pressure, the extent of adsorption grows less fast with increasing pressure, and hence the graph for medium pressure is shaped like a curvature.
At high pressure
When the pressure is high, 1/n = 0.
x/m = a constant
In this case, the extent of adsorption is independent of pressure, and the graph is therefore parallel to the pressure axis at high pressure.
Nature of Adsorbent
Because adsorption is a surface phenomenon, the number of adsorption increases according to the amount of surface area of the adsorbent. The higher the surface area of an adsorbent, the finer the surface division or the rougher the surface texture. as a result of which the adsorption will be larger. Metal catalysts in finely divided form, colloidal form, rough surfaces, and activated adsorbent provide a greater surface area than other forms of metal catalysts.
Chemisorption is more selective and preferred than adsorption. Gas will only be chemisorbed on a solid if the solid has a wide surface area that can accommodate the gas. For example, nickel can absorb hydrogen gas but not iron, but iron cannot. Adsorbents should have a chemical composition that allows them to cause chemisorption of adsorbate on them.
Nature of Adsorbate
As a result of stronger van der Walls forces, it has been discovered that more easily liquefiable and highly water-soluble gases are adsorbed more readily by solids when the adsorption of gases by solids is investigated. To do this, ammonia, hydrogen chloride, chloride, and sulphur dioxide are adsorbed at a higher rate than hydrogen, nitrogen, and oxygen
When it comes to physical adsorption, the amount of adsorption is determined by the boiling point of the gases involved. Gases are more readily adsorbed on solids than they are on liquids.
The concentration of Adsorbate
Generally, when liquid is adsorbed on solid, the extent of adsorption is greater at higher concentrations of adsorbate, as long as the temperature is maintained at a constant level. The concentration of adsorbate has a comparable impact to that of pressure in terms of its effectiveness.
Adsorption Isotherm
Adsorption isotherms are defined as the relationship between the extent of adsorption (the amount of a substance adsorbed per unit mass of an adsorbent) and the equilibrium pressure or concentration at a constant temperature under uniform conditions. At a fixed temperature and pressure, it is a curve generated by graphing the extent of adsorption (the amount of a substance adsorbed per unit mass of an adsorbent) versus the equilibrium pressure or the concentration of the substance adsorbed.
Freundlich Adsorption Isotherm
Adsorption isotherms are curves that are created by graphing the extent of adsorption (the amount of a material adsorbed per unit mass of an adsorbent) against the equilibrium pressure or concentration while maintaining a constant temperature.
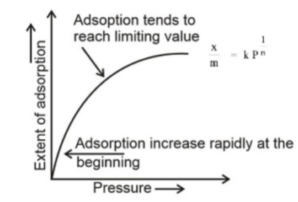
It is known as the “Freundlich adsorption isotherm” since it was proposed by Freundlich and is an empirical equation for the variation of gas adsorption with pressure at a constant temperature over a narrow range of pressure. The equation is as follows in terms of mathematics:
X/m = kP1/n or x/m=kC1/n
Where x denotes the mass of adsorbate (gas) that has been adsorbed.
m is the mass of the adsorbent (solid).
P represents the equilibrium pressure of the adsorbate.
The concentration of adsorbate in solution at equilibrium is denoted by C.
In this equation, the variables ‘K’ and ‘n’ are constant, and the variable ‘n’ is less than one.
Conclusion
It is the process by which a substance (referred to as the adsorbate or the sorbate) is accumulated on the surface of a solid (adsorbent, or sorbent). The adsorbate can exist in either a gaseous or a liquid state. It is unsaturated forces at the solid surface that act as a driving force for adsorption and have the potential to establish bonds with the adsorbate. Adsorption processes that take place on cell membranes stimulate a wide range of important chemical reactions while also causing changes in surface tension and cell consistency, among other effects. Adsorbed drugs and poisons can exert their effects on cell surfaces because of their placement on the cell surface. Selective adsorption may be related to a specific activity. Specifically, the surface area of the adsorbent is directly proportional to the extent of adsorption; that is to say, the bigger the surface area of the adsorbent, the greater is the amount of adsorption. The surface area of a powdered solid adsorbent is determined by the size of the particles in the adsorbent. The higher the surface area of a particle, the smaller the particle size.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






