Equilibrium is a condition of balance or a stable situation in which opposing forces cancel each other out and no changes occur. In economics, equilibrium occurs when supply and demand are equal. When you are peaceful and stable, this is an example of Equilibrium.
Chemical Equilibrium
Chemical equilibrium is a dynamic state in which all reactant concentrations remain constant. They aren’t equal, but they aren’t going to change. A double arrow in a chemical process denotes a state of equilibrium. On the left are reactants, while on the right are products.
The system achieves chemical equilibrium when the rate of the forward reaction is equal to the rate of the reverse reaction. The system is considered to be in a state of dynamic equilibrium when the concentrations of the reactants and products do not vary due to the equal rates of forward and reverse reactions
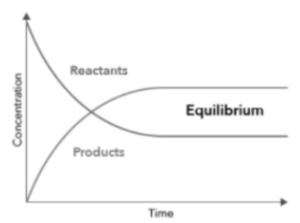
A graph can be shown with the concentration on the y-axis and time on the x-axis. Chemical equilibrium is reached when neither the reactants’ nor the products’ concentrations change.
Chemical Equilibrium As “Dynamic Equilibrium”
An equilibrium stage is defined as the point where the rate of forward response equals the rate of backward reaction. The number of reactant molecules changing to products and product molecules turning to reactants is the same at this moment. Chemical equilibrium is dynamic because the same equilibrium can be achieved with the same reactants in identical conditions anywhere with ongoing molecule exchange.
Different Types Of Chemical Equilibrium
Chemical equilibrium can be divided into two categories:
1.Homogeneous Equilibrium
2.Heterogeneous Equilibrium
Homogeneous Equilibrium
The reactants and products of chemical equilibrium are all in the same phase in this kind. The two types of homogeneous equilibrium are as follows: The number of molecules in the products equals the number of molecules in the reactants in these reactions.
For instance,
H2 (g) + I2 (g) = 2HI (g)
and
N2(g) + O2 (g) = 2NO (g)
Reactions in which the total number of reactant molecules is greater than the total number of product molecules.
2SO2 (g) + O2 (g) = 2SO3is an example (g)
CO (g) + Cl2 (g) = COCl2(g)
Heterogeneous Equilibrium
The reactants and products of chemical equilibrium are present in distinct phases in this type. Here are a few examples of heterogeneous equilibrium.
2CO = CO2(g) + C (s) (g)
CO2 + CaCO3 (s) + CaO (s) (g)
The phase of the reactants and products determines the distinct types of chemical equilibrium.
Following Factors Affect The Chemical Equilibrium
1.Change In Concentration
2.Change In Pressure
3.Change In Temperature
4.Addition Of a Catalyst
If any of the components determining the equilibrium circumstances change, the system will counteract or minimise the influence of the overall transformation, according to Le-Chatelier’s Principle.Both chemical and physical equilibrium are governed by this principle.
The temperature, pressure, and concentration of the system are all factors that affect equilibrium.
Some Of The Examples Of Chemical Equilibrium
Reactants are turned into products in chemical reactions by the forward reaction, while products can be converted back into reactants by the reverse reaction. The composition of the two states, reactants and products, differs.
The pace of forward and backward reactions may become equal when a certain amount of time has passed after the reaction began. The amount of reactants converted will then be created again by the reverse reaction, resulting in no change in the concentration of reactants and products. As a result, the reactants and products are chemically balanced.
N2+H2=2NH3
PCl5=PCl3+PCl2
Importance Of Chemical Equilibrium
It’s used in a variety of industrial activities, including
1.Haber’s process for producing ammonia: When nitrogen reacts with hydrogen to generate ammonia, the yield is higher at low temperatures, high pressures, and using iron as a catalyst.
2 Sulphuric acid is made through a contact procedure. The oxidation of sulphur dioxide to sulphur trioxide is the primary reaction in this process. Chemical equilibrium is required for this.
Conclusion
The equilibrium state is one in which the concentrations of reactants and products do not fluctuate. Even if there is no visible change in equilibrium, this does not mean that all chemical reactions have stopped.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






