Alkenes are branched or unbranched acyclic hydrocarbons with one carbon-to-carbon double bond (C=C) and the typical chemical formula CnH2n. Alkenes are unsaturated because they have very few hydrogen atoms per carbon atom than the maximum number conceivable. Olefins is another word for alkenes which is still used in the petroleum industry.
Although the names olefins and alkenes are sometimes used interchangeably, this is not entirely correct. Alkenes are all aliphatic hydrocarbons with one and only one double bond, according to IUPAC. In reality, all the aliphatic (both acyclic and cyclic) hydrocarbons having one or more carbon-to-carbon double bonds are classified into olefins: alkenes, cycloalkenes, and polyenes (compounds exhibiting more than one double bond). The nomenclature changes to alkadiene, alkatriene, and so on when an alkene contains more than one double bond. As pyrolysis products of certain polymers, alkadienes are frequently found in fire debris analysis.
Alkene Structure
The Alkenes have a double bond between the two carbon atoms which is made up of one pi and one sigma bond. The characteristics of the sigma bond are similar to those of alkanes, whereas the pi bond is more reactive. The double bond’s carbon atoms are sp2 hybridized, resulting in a planar structure. Because rotation around the double bond is undesirable, alkenes form relatively stable isomers when substituents are placed on the same (cis) or opposite (trans) sides of the double bond. Diastereoisomers are said to be this type of isomers.
EthyleneA space-filling model of the ethylene, simplest alkene, showing its planar structure.
Physical Properties of Alkene
The regularity of the packing, or the proximity, of these molecules determines the melting and boiling points of alkenes. Higher melting and boiling points are found in alkene isomers that can achieve more regular packing than molecules with the same molecular formula but weaker dispersion forces. The Non-polar alkenes are immiscible in the water and they have a lower density than water. Usually, they are soluble in organic solvents. Furthermore, they are not conductors of electricity.
Chemical Properties of Alkenes
Alkenes are unsaturated chemicals with a high degree of reactivity. Because of the presence of a double bond, the alkenes are more reactive than alkanes. The σ-connection is powerful, yet π- it is also weak. Alkene reactions typically include the insertion of an electrophile across the double bonds via an ionic process. Additional reactions, on the other hand, use the free-radical mechanism. Alkenes undergo a variety of reactions, including ozonolysis and polymerization.
Addition Reaction
Addition of hydrogen: (Hydroge-nation of alkenes)
In the presence of a metal catalyst (Ni, Pd, or Pt), hydrogen is added to alkenes to produce equivalent alkanes. Catalytic hydrogenation is the term for this process. This step is critical in the production of vanaspati made from vegetable oil. This helps to keep vegetable oils from becoming rancid.
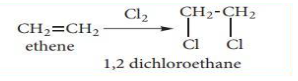
Addition of halogens: (Halogena-tion of alkenes)
When an alkene is exposed to halogens such as chlorine or bromine, addition occurs quickly, resulting in the formation of 1, 2- dihalo alkane (or) vicinal dihalide.
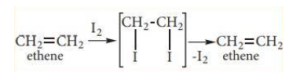
Iodine interacts slowly to make 1, 2 – diiodo alkanes, which are unstable and can be regenerated by removing the iodine.
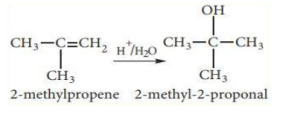
Addition of water: – (Hydration of alkenes)
Water does not normally react with alkenes. Alkenes react with water to form alcohols in the presence of concentrated sulphuric acid. The reaction follows Markovnikoff ’s rule and carbon-cation mechanism.
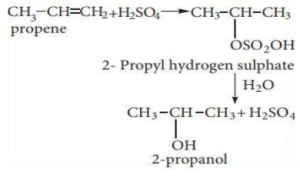
Addition of Sulphuric Acid to Alkene
According to Markownikoff’s rule, alkenes react with cold and concentrated sulphuric acid to create alkyl hydrogen sulphate. Alcohol is produced by further hydrolysis.
Oxidation
(I) With cold dilute alkaline KMnO4 solution (Baeyer’s Reagent)
Vicinal diols are formed when alkenes react with Baeyer’s reagent. The purple solution (Mn2+) changes to a dark green (Mn6+) and ultimately to a dark brown (Mn4+) precipitate.
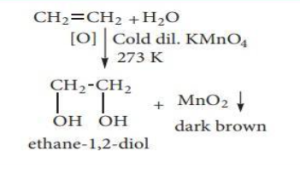
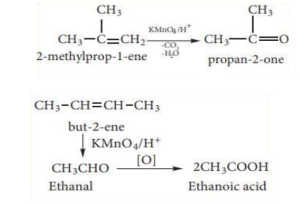
(ii) With acidified KMnO4 Solution:
Alkenes are oxidised to ketones or car-boxylic acid when they react with an acidified KMnO4 solution, depending on the substituent at the olefinic carbon atom. Purple solution loses the colour and becomes colourless. This is one of the unsaturation tests.
Reactivity of Alkene
Due to the increased fragility of the double bond, alkenes are more reactive than their related alkanes. They are more prone to take part in a wide range of processes, such as combustion, addition, hydrogenation, and halogenation. Alkenes can also be reacted to produce polymers, usually in the presence of a catalyst.
Classification Of Alkene
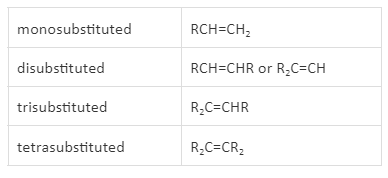
The stability of double bonds in alkene is affected by the alkyl groups attached to sp2 hybridised carbon atoms of the alkenes. The quantity of alkyl groups linked to the sp2 hybridised carbon atoms can also alter the chemical reactivity of alkenes. As a result, alkenes can be classified according to the number of alkyl groups connected to the C=C structural unit. The degree of substitution is the term for this attribute.
Monosubstituted alkenes have a single alkyl group linked to the sp2 hybridised carbon atom of the double bond. A terminal alkene is an alkene with its double bond at the end of the carbon atom chain. Disubstituted, trisubstituted, and tetrasubstituted alkenes consist of two, three, or four alkyl groups attached to carbon atoms of double bond, respectively.
Conclusion
Alkenes, commonly known as olefins, are organic molecules that have one or more carbon-carbon double bonds in their chemical structure and are made up of carbon and hydrogen atoms. Unsaturated hydrocarbons are known as alkenes. They are hydrocarbons because they are made up entirely of carbon and hydrogen atoms, and they are unsaturated because their chemical structure contains one or more double bonds.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out