Solubility is a state of being where one substance is soluble in another substance. It can also be defined as an ability of a solute to dissolve in the solvent. The solubility of a substance is often influenced by the solvent used, temperature and pressure. The degree of solubility increases from infinitely soluble to poorly soluble. When additional solute added to the solvent does not increase the concentration of the solution, it is called a saturated solution.
Solubility product
Solubility product is defined as the equilibrium constant at which solute dissolves in a solvent or aqueous solution. It is denoted by Ksp. The solubility product of a solution is constant at a constant temperature. If the solubility product is higher, then the solubility of the solution is higher and if the constant is lower, so as the solubility will be. The saturated solution of ionic compounds has less solubility. Molar solubility is related to solubility products, but they are not similar.
Factors affecting solubility product constant
There are 3 different factors that affect the solubility product :-
- Effect of Ion Pairs– Ion pairs are represented by a cation and an anion. When these ion pairs are involved in pairing, then the Ksp value is lower than the experimental value. So, to meet the Ksp value, more solute needs to be added.
- Common Ion effect- According to Le Chatelier’s principle or equilibrium law, if the concentration of ions is excess, then these excess ions must be removed by combining them with oppositely charged ions. So, in the common ion effect, the ionisation is suppressed, and the solubility is decreased by the common ion. At a given equilibrium, the presence of a common ion reduces the Ksp value, and the absence of a common ion has a greater Ksp value.
- Salt effect or Diverse ion effect- The presence of uncommon ions that are not involved in the equilibrium increases the Ksp value. This has an opposing effect as compared to common ions.
Solubility product formula (Ksp)
The solubility product constant is denoted by Ksp where,
K= constant
sp= solubility product
Here, we will take a general reaction example,
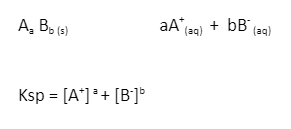
Example-
1) The expression for solubility product of Calcium fluoride-

2) The expression for dissolution of barium sulphate-
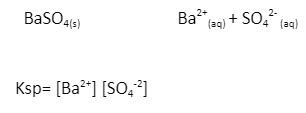
Significance of Solubility product
- Lattice enthalpy of salts– Under standard conditions, the amount of energy required to form one mole of ionic lattice from gaseous ions is called lattice enthalpy. The strong attraction of salts (lattice enthalpy) is suppressed by the solvent interactions with ions when it dissolves in a solvent.
- Solvation enthalpy of ions- Solvation enthalpy is the change in enthalpy minus the product of the temperature times the change in entropy. The solvent determines the amount of energy released during solvation enthalpy. If the reaction results in negative solvation enthalpy, then it depicts that energy is released during the process.
The non-polar solvents do not have sufficient energy for overcoming the lattice enthalpy, so they have low solvation enthalpy. During solvation, the nature of the solvent is determined to find out the amount of energy released. For salt to be dissolved in a given solvent, it must have a solvation enthalpy that is greater than lattice enthalpy.
Conclusion
The solubility of different solids varies a great deal. Some solutes are highly soluble, while others of them are poorly commonly termed insoluble. When the rate of dissolution is similar to the rate of crystallisation, then the solution is said to be in equilibrium. The equilibrium concentration is denoted by the product of the concentration of ions in the solution.
A substance’s solubility is determined by the solvent employed, temperature and pressure. The solubility product is applicable for salts that are sparingly soluble in water. Each salt has its characteristic solubility, which depends on the temperature. So, as per the theory, we come to know that the solubility product remains constant at a given constant temperature. Temperature is one of the crucial factors affecting compound dissociation. Under certain reaction conditions, the equilibrium solubility surpasses and comes up with a supersaturated solution.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out












