Chemistry involves multiple chemical reactions classified based on the type of mechanism and involving reagents. One such type of popular chemical reaction is the elimination reaction. It is the reaction that completes the substitution reaction. One equivalent of acid is replaced by an alkyl halide and eliminated in the elimination reaction.
Let us cover all the topics related to this reaction in this section of the study material. We’ll start with the definition of elimination reaction and its examples. We’ll also cover the detailed mechanism of these reactions and the law governing the formation of products in the elimination reactions.
Elimination reaction- A quick review
The reaction in which organic compounds having single carbon-carbon bonds are transformed into compounds having double or triple carbon-carbon bonds can be termed an elimination reaction. One of the main applications of the elimination reactions is the preparation of alkenes.
Elimination reaction example
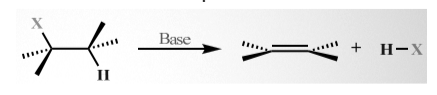
The formation of double or triple carbon-carbon bonds can be achieved in two different ways: the two mechanisms of elimination reaction.
Elimination reaction mechanism
The two elimination reaction mechanisms are:
1.E2 mechanism: In this mechanism, bimolecular elimination takes place. All the dehydrohalogenation reactions are based on the E2 mechanism only. A simple example of the E2 mechanism is:
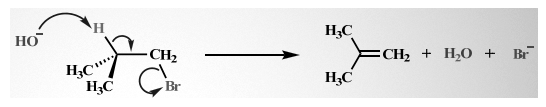
This reaction follows the second-order kinetics, and the base with the alkyl halide is formed. Hence, the rate equation becomes rate = k [(CH3)3 CBr] [HO–]. The reaction thus taking place is concerted and hence has all bonds created in a single step only.
Characteristics of E2 reaction
- It has second-order kinetics.
- It has a one-step mechanism only.
- The most substituted halides react faster- R3CX> R2CHX> RCH2X.
- Stronger bases favour the strength of the base.
- The better leaving group leads to quick reaction rates.
- The solvents used are the polar aprotic ones.
Factors affecting the rate of E2 reaction
It differs in the way how the R group affects the reaction rate. Hence, with the increase in the R groups on the carbon within the leaving group, the rate of E2 reaction increases. Further, the E2 reaction rate increases with the alkyl substitution, which can be rationalised with the transition state stability.
2.E1 mechanism:
In this mechanism, unimolecular elimination takes place. E1 mechanisms follow a two-step mechanism, which involves one leaving group bond-breaking first and forming the pie-bond after it. A simple example of the E2 mechanism is:
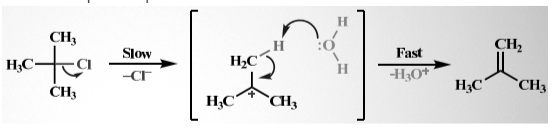
This reaction follows the first-order kinetics, and the base with the alkyl halide is formed. Hence, the rate equation becomes rate = k [(CH3)3 CCl].
Characteristics of E1 reaction
- It has first-order kinetics.
- It has a two-steps mechanism.
- The most substituted halides react at a faster rate- R3CX> R2CHX> RCH2X
- Weaker bases like H2O or ROH favour the strength of the base.
- The better leaving group leads to quick reaction rates. For example, in the SN1 reaction, the C-X bond cleavage is the foremost rate-determining step.
- The solvents used are the polar protic ones that stabilise the ionic intermediates.
Factors affecting the rate of E1 reaction
The rate of E1 reaction depends on the presence of R groups on the carbon within the leaving group. With the increase in the R groups, there is an increase in the rate of E1 reaction increases. Further, the acid’s strength defines the type of mechanism followed in the reaction- E1 or E2 reaction. The bases like H2O, ROH, etc., favour E1 reactions.
Conclusion
Hence, it is easy to understand the mechanism of elimination reactions. The two types of mechanism, i.e., E1 and E2 reactions, can be understood quickly with a detailed understanding of the molecule displacements. The rules governing the formation of final products in elimination reactions are Satyzeff’s rule.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out













