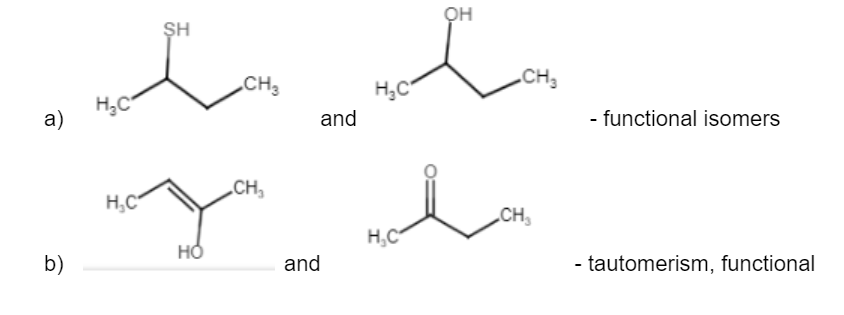
Isomerism is an important concept in organic chemistry that is responsible for giving unique identity to similar compounds. There are two types of isomerism – structural isomers and stereoisomers, each with their own specialties and types. Read on to know more about structural isomerism and its applications.
What are Structural Isomers?
Structural isomer (or constitutional isomers) of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. For example, Ethanol [CH3CH2OH] and Methoxymethane [CH3-O-CH3] have the same chemical formula – C2H6O but then their bondings are different as is visible from the above formulae. This also applies to polyatomic ions with identical total charges. For example, let us consider the cyanate ion [O=C=N] and the fulminate ion [C–=N+O]. It can also be extended to ionic compounds. For example, ammonium cyanate [NH4CNO] and urea [CO(NH2)2] are structural isomers too.
There are various types of structural isomerism.
- Chain isomerism
- Positional Isomerism
- Functional Isomerism
- Metamerism
- Tautomerism
Let us use structural isomers examples to understand each of these classifications.
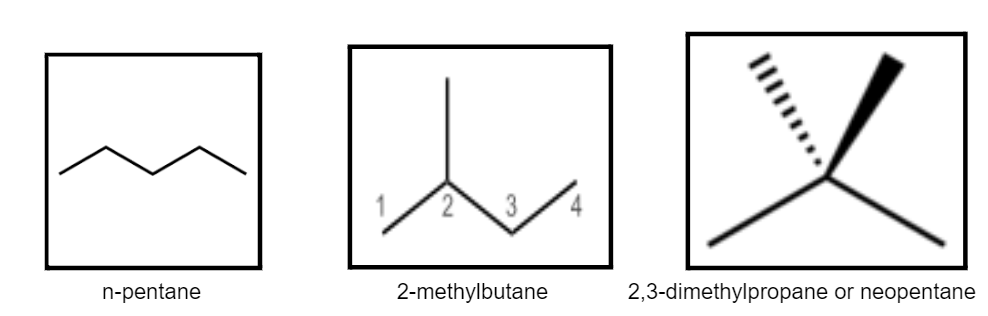
Chain Isomerism
This is also known as skeletal isomerism. Chain isomerism arises due to the difference in the arrangement of carbon chains within the molecule. Two compounds having the same molecular formula have a different main chain, then they exhibit skeletal or chain isomerism. To understand the concept, let us look at the example of pentane or C5H10.
Positional Isomerism
This type of structural isomerism arises when the same functional group is located at different positions on identical carbon skeletons of compounds that are isomers. To understand it better, let us look at the following example of the compound with the molecular formula C3H6O.
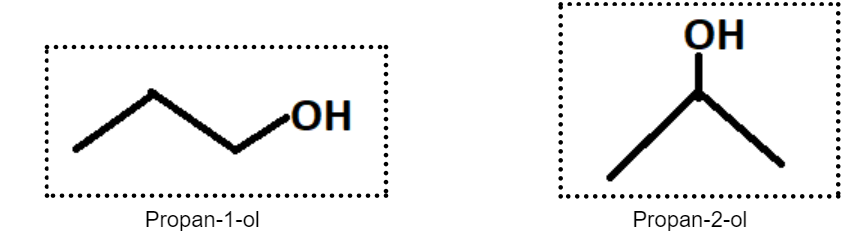
Thus, it becomes clear how positional isomers work,i.e., if in two compounds having identical formulae, one or more of the functional groups are located at different sites on the carbon skeletal chain, then they are known as position isomers.
Functional Isomerism
Compounds that have the same molecular formula but have different functional groups attached to them are said to show functional isomerism, or that they are functional isomers. To have a better understanding of what functional isomerism is let us look at the following example – let us consider the molecular formula C3H6O. Three structures may be drawn for this:
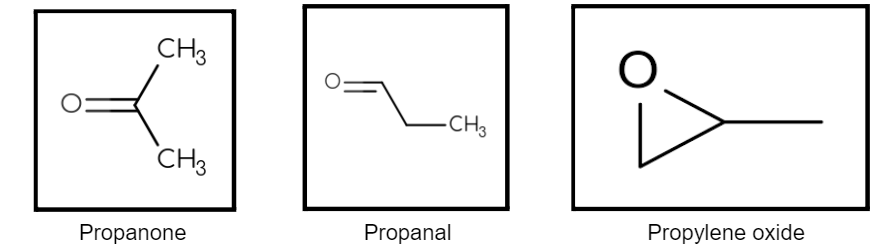
Thus it is seen that from the same molecular formula, three compounds can arise having different functional groups – a ketone, an aldehyde, and an epoxide. From this, we observe that for the same molecular formula, we get three compounds having different functional groups and belonging to their respective homologous series. These three compounds are functional isomers as they have different functional groups but the same molecular formula.
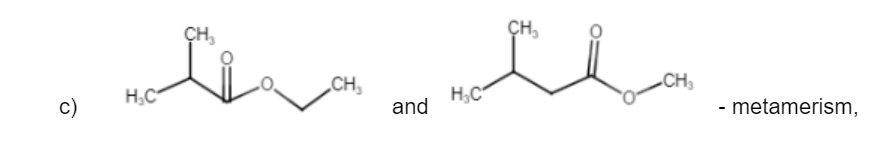
Metamerism
If two compounds have the same molecular formula but have different alkyl groups on the two different sides of the functional group, then they are said to be metamers and the type of structural isomerism shown by these two compounds is known as metamerism. If we consider the molecular formula C4H10O, then we can have two different structures for them –
The alkyl chains on both sides of the O-atom are different and these two compounds are metamers. This type of isomerism is also shown by esters and ketones. You can read more about them in books on isomerism or from the web.
Tautomerism
It is a phenomenon where a chemical compound tends to exist in two readily interconvertible forms which differ usually in terms of the nucleus of one atom, usually hydrogen.
The two forms are usually in a state of dynamic equilibrium and convert into each other depending upon a variety of conditions – for example, pH of the solution or depending on the presence of other substances near them. Usually, the stability of one form is greater than the other and the compound tends to exist in that state predominantly. It can however be converted to the other form easily for reactions or for other purposes easily. Let us see the example of a compound with the molecular formula C3H6O to understand how tautomerism works.

As we can see that the two structures are tautomers to each other as they differ only in the relative position of the hydrogen atom and the double bond present. For more information insult various literature available throughout the web or books.
Applications of Structural Isomers
Structural isomers having the same molecular weight and formula but with different structures. Due to this difference in bonding, two isomers can show a variety of differences if we consider their physical and chemical properties. This is made use of in many chemical industries, specially in the petrochemical ones. They also find some usage in the biochemical and medical industries too. They are also used to obtain a compound to which they are a precursor, so that another preparative pathway would not have to be designed. They are extremely important for determining the structures and hence the properties of a compound as changes in structure would imply significant changes to bondings and other interactive forces around the molecule. They also find usage as solvents in studying particular reactions.
Conclusion
If you are interested in studying organic chemistry, isomerism plays a very important role in every reaction that you will come across. Learning to draw structural isomers and noticing the differences between two isomers is extremely important and you would come across it from time to time. Structural isomerism is an extremely important part of organic chemistry and is one of the bases as to why it is so interesting to figure out and use the compounds for our purposes.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out