Isomerism is an important concept in organic chemistry that is responsible for giving unique identity to similar compounds. Isomers are of two different kinds – structural isomers and stereoisomers. They have their own specialities, kinds, and applications. In this article, we are going to talk about one of the kinds of isomers – stereoisomers. Read ahead to know more about stereoisomers and their applications.
What are Isomers?
In organic chemistry, isomers are compounds that have the same chemical formula but differ in properties and the arrangement of atoms in the molecule. They basically are molecules that have the same molecular formula but show different chemical and physical characteristics. Isomers in organic chemistry are divided into two major parts – structural isomers and stereoisomers. Both of them can be further divided into various subparts. In this article, we will learn more about stereoisomers, their classifications and their applications. In stereoisomers the bondings of the atoms appear to be the same yet their orientations in space are different.
What are Stereoisomers?
Stereoisomers, also known as stereoisomers, are molecules or compounds that have the same formulae and molecular bondings but have different spatial arrangements of atoms in 3D space. They differ from structural or constitutional isomers as the bonding connectivities remain the same, only the spatial arrangement of atoms is different.
In order to gain a better understanding of what stereoisomers are, let us consider the example of two buildings built with the same material and have the same number of floors yet they differ in how the parts are connected. Thus they form the example or reference to constitutional isomers. Now, if we consider the floor plans of the two houses and compare them and we see that they are mirror images of each other (builders sometimes do that in order to make the houses look different from the street), then they would be stereoisomers.
There are basically two types of stereoisomers – geometrical isomers and optical isomers, which can also be divided into subparts. We shall learn more about them as we proceed.
Geometrical Isomers
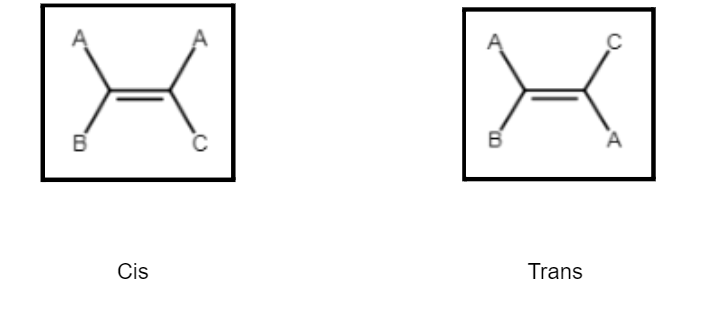
We know that atoms can rotate freely about a single bond. However, the rotation can be restricted by replacing the single bond with a double bond. Thus there would be restricted rotation because the breakage of the bond would mean compromising the stability of the molecule. Since the bonds cannot rotate, they give rise to shapes that cannot interconvert. So geometrical isomerism is a type of stereoisomerism having the same molecular formulae and connectivities but differs in the relative arrangement of atoms. It arises due to different possible geometrical arrangements of the ligand. Geometrical isomers are usually of two types – cis and trans (or E and Z). Let us look further into it with the help of an example:
Consider the example of but-2-ene [ CH3CH=CHCH3]. This can have two geometrical isomers – the cis and the trans forms as follow:
Since the atoms cannot rotate about the double bond, thus we see that there would be two arrangements of the groups possible. This gives rise to two geometrical isomers – the cis and the trans forms. Let us learn what these terms signify and how to identify them:
If the formula of the molecule is of the type AAC=CXX, then:

If the formula is of the type AAC=C(B)(C):
Optical Isomerism
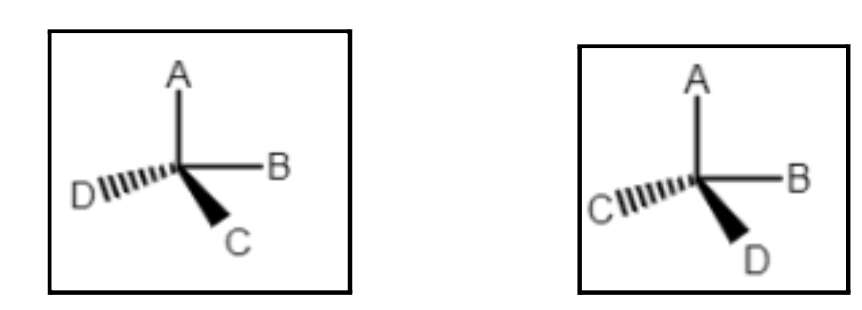
Molecules which have similar molecular weight and physical characteristics but differ in their behavior towards the rotation of plane polarized light. Optical isomers have the same structure and formulae but they cannot be superimposed on each other, i.e., they are mirror images of each other. It can be found only in compounds that have an asymmetric carbon center; in order words, there must be four different groups attached to a carbon center. They can turn either plane-polarized light towards the left or right and are termed as d- or l- isomers.
If there are four different groups attached to the carbon atom, then it is said to be an asymmetric carbon center. Such optically active compounds are said to be chiral.
Applications of Stereoisomers
Stereoisomers find a wide variety of applications. Most reactions in higher organisms are stereospecific which means that only one of the enantiomers can react whereas the other cannot. Also some enantiomers of a compound are harmful and maybe lethal whereas the other enantiomer of the same molecule is beneficial. It is widely used in drug synthesis and application as only one of the enantiomers is beneficial. It is also used in the development of chemicals where only one specific enantiomer is required and processes are designed using only one of the enantiomers. Thus optical isomerism as well as geometrical isomerism find a huge amount of usage in the research and practical world.
Conclusion
Thus to conclude, there are several types of applications of isomers, especially optical isomers in today’s world. Learning to draw and figure out optical isomerism is a very important part if you are studying chemistry or biochemistry-related subjects. They are extremely important in helping scientists to figure out the various biochemical reactions happening in our bodies and this helps them to develop various drugs and medicinal techniques. Thus stereoisomerism is extremely important in these fields.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out












