Different types of chemical reactions are used in chemistry. When it comes to specific reactions, elimination reactions are prevalent in multiple chemical applications. A Russian chemist named Alexander Zaitsev has analyzed different elimination reactions and has given Saytzeff’s rule.
Let us learn all about Saytzeff’s rule, its examples, and its working mechanism. In this section, we’ll make it easy for the students to understand the nature of alkyl halides and alcohols. We’ll learn the basics of the elimination reaction and its aim to create a stable product in the reactions.
Elimination reaction
The organic chemical reaction in which two elements are removed from a molecule in a single-step or double-step mechanism is termed an elimination reaction. Based on the bimolecular kinetics of the reaction, the single-step or double-step mechanisms are categorized as E1 for two-step mechanisms and E2 for one-step mechanisms.
When the molecule has a poor leaving group but can stabilize the anion, the third type of elimination reaction- E1CB takes place. The reaction rate in the elimination reaction depends on the reactivity of different participants like bromide, iodide, or halogens. When talking about Saytzeff’s rule, it applies to the elimination reactions only.
Dehydrohalogenation
The elimination reaction is when a specific hydrogen halide is removed from the substrate. The dehydrohalogenation is linked to the alkenes synthesis but can be used for other chemical compounds. The ideal substrates for dehydrohalogenation reaction are alkyl halides. Due to the requirements to form alkene while undergoing dehydrohalogenation, benzyl halides and methyl halides are not suitable substrates for the reaction.
Some of the other dehydrohalogenation reactions include isocyanides, epoxides, base-promoted reactions to alkynes, base-promoted reactions to alkenes, etc. Some exclusive reactions include chlorobenzene dehydrohalogenation treatment with a solid base to give benzyne intermediate and phenol. Saytzeff’s rule determines the type of mechanism in dehydrohalogenation reactions.
Saytzeff rule with an example
Saytzeff’s rule states that “in any dehydrohalogenation reaction, the elimination can occur in two different processes. Hence, the alkene’s preferred product has the greatest number of alkyl groups linked to the doubly bonded carbon atoms.”
It is also called Saytzev’s rule or Zaitsev’s rule and offers an empirical formula to detect the preferred alkene products in any elimination reaction. A Russian chemist Alexander Zaitsev established this, worked on different elimination reactions at University in Kazan and established the generalized trends. The trend was positive for the alkene formations, which resulted in the removal of hydrogen from the alpha-carbon hydrogen substitutes.
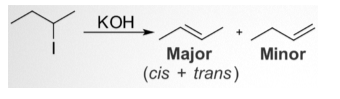
In this chemical reaction, 2-iodobutane is treated with alcoholic potassium hydroxide or KOH to form 2-butene as the significant product and 1-butene as the minor product. Hence, Saytzeff’s rule works on the regiochemistry of the elimination reaction while ignoring the generalizations about the stereochemistry of the newly formed alkene.
Saytzeff’s rule exceptions
Like any other rule aiming at the categorization of the chemical reactions, Saytzeff’s rule is not applicable to certain products. Hofmann products are the compounds having quaternary nitrogen and leaving groups like SO3H, NR3+, etc. These products are valid exceptions to Saytzeff’s rule.
The amount of energy released during the hydrogenation reaction is considered while considering the stability of the Saytzeff products. The stable alkenes have lower heat of hydrogenation, and hence it becomes easy to determine the thermodynamics stability of the reaction.
Conclusion
Hence, it is easy to understand the preferred way of the elimination reaction when it comes to the alkene products formation. Saytzeff’s rule states that in any dehydrohalogenation reaction, the select product is the alkene having a large number of the alkyl groups attached to the doubly bonded carbon atoms in the reaction.
A quick look at the Saytzeff rule with examples makes it easy for our students to memorize and understand this important rule for elimination reactions. Not to miss is the top asked questions on Saytzeff’s rule, which ensures that students don’t have to look here and there for their possible queries.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out












