Introduction:

Physical Properties:

Chemical Properties:
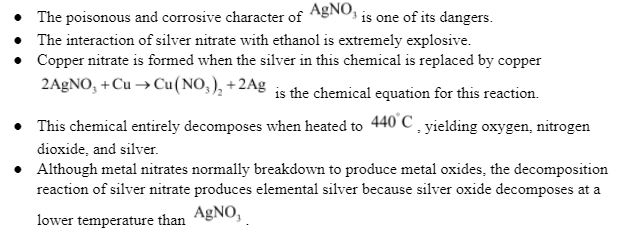
Preparation:
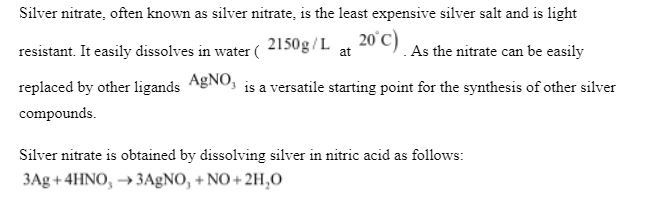
Adverse Health Effects of Silver Nitrate:
- Silver nitrate is a corrosive substance. As a result, this will irritate you. It creates a greyish staining of tissues when it is absorbed in the skin over a long period of time. The term for this phenomenon is argyria.
- Mucous membranes will be irritated and damaged if this is breathed.
- When Silver Nitrate is used by mouth, it can have significant side effects and could result in death. Vomiting, bloody diarrhoea, dizziness, and seizures are all possible side effects.
The following are some of the long-term impacts of Silver Nitrate exposure:
- It can lead to feelings of sadness, headaches, and a decrease of mental abilities. Cancer has also been observed in certain people who have been exposed to Silver Nitrate.
- When someone comes into touch with Silver Nitrate, they must wash it off with a substantial amount of freshwater. Medical attention should be given to them right away.
- Fish and other aquatic life forms are likewise very poisonous to silver nitrate. As a result, disposal of this chemical should also be done in accordance with local laws and regulations.
Uses of Silver Nitrate:
- Silver nitrate is used in a variety of domains, including biology, chemical synthesis, and medicine. The following are a few of the applications.
- Because the nitrate ion can be replaced by different ligands that can bind to the silver ion, silver nitrate is an extremely versatile molecule.
- This chemical is employed in the production of photographic films because it can generate a precipitate of silver halides when exposed to halide ions.
- A precipitation reaction of silver nitrate can be used to make a variety of silver-based explosives. This molecule is used to extract halides in the field of inorganic chemistry.
- This reaction is used in analytical chemistry to check for the presence of halide anions like iodide, bromide, or chloride ions.
- Because the silver cation bonds with alkenes in a reversible manner, this molecule can be used to separate mixtures of alkenes.
- Silver nitrate, when diluted with water to a concentration of 0.5 percent, can be used as an antiseptic in a variety of medical settings.
- A diluted solution of AgNO3 can be injected into the eyes of a newborn born to a gonorrhea-infected mother, which kills the gonococcal bacteria and protects the child from blindness.
Conclusion:
AgNO3 is the chemical formula for silver nitrate, an inorganic substance. This salt can be used to make a variety of silver compounds, including those used in photography. It is far less light sensitive than halides. It was originally known as lunar caustic because ancient alchemists associated silver with the moon and termed it luna. The silver ions are three-coordinated in a trigonal planar configuration in solid silver nitrate.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out











