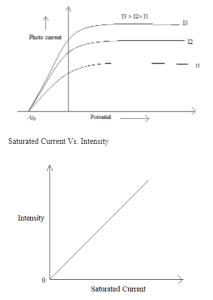
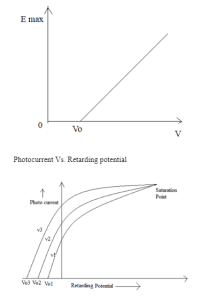
In the year 1887, a physicist of German named Heinrich Rudolf Hertz discovered the photoelectric effect was. The photoelectric effect is a phenomenon in which when any kind of electromagnetic radiation hits the metallic surface or surface of the metal, the ejection or emission of electrons occurs. Photoelectrons are the electrons that are emitted by the emission of electrons on a metal surface. It is an instantaneous or spontaneous process.
Einstein’s Photoelectric Effect
Einstein’s Photoelectric Effect is defined in the following two ways when a photon of energy hν incidents on a surface of the metal, the energy of the photon is absorbed or consumed by the electrons –
- a) The work function is a part of the energy that is used to overcome the surface barrier and emission of electrons from the metal surface. Work function is denoted by φ0 and is expressed as φ0 = hν0
- b) The part of the energy which is remaining is used for proving a velocity to the photoelectron, which is equivalent to the maximum kinetic energy. The energy of a photon is given when the incidents of frequency of the photon are more than that of the threshold frequency. According to the law of conservation of energy
hν= φ0 + ½ mv2 ……………. Equation (a)
φ0 = Work Function
ν = Incident photon’s frequency
h = Plank’s Constant = 6.6261 x 10-34 Js
E = hν ……….. Equation (b)
E = the ejected electron’s maximum K.E.
hν = φ0 + K.E. max ………. Equation (c)
[It is known that ½ mv2 = K.E. max]
If any photon is incident to threshold frequency then, hν0 = φ0
Φ0 = hν0 ………… Equation (d)
From the Equation (c) and (d)
hν = hν0 + K.E. max
- K.E. max = hν – hν0
By taking ‘h’ as common from hν and hν0
We get,
K.E. max = h (ν – hν0)
Therefore, this is Einstein’s Photoelectric Effect, and the equation
Experimental study of the photoelectric effect
The process of photoelectric emission is defined as the emission of electrons from the metal surface when incident light falls on the metal surface. Without any time lag, the photoelectric emission process is a spontaneous process
The figure above shows the experimental setup for the study of the photoelectric effect. The figure contains a vacuumed glass tube, two metal plates ‘A’ and ‘C’, a microammeter is placed in the circuit – which indicates the current, a potential divider, or a rheostat is present which can be moved forward or backward for gathering the knowledge about the potential difference between the two plates ‘A’ and ‘C’, there is s switch present in the circuit along with the battery. Here, ‘A’ is the plate which is marked as the collector and ‘C’ is the plate that is marked as the ammeter. The window ‘W’ which is transparent is sealed on vacuumed glass tube which can be covered with a filter for light for particular radiation, which will, as a result, allow the light of a particular wavelength to pass through it. The emission of the electron will allow the photoelectric current to move in a circuit. A plate ‘A’ can be provided with desired positive or negative potential concerning plate ‘C’ using the arrangement of a battery and a microammeter is connected to measure the photoelectric current in the circuit.
Working principle of the Experimental study of the photoelectric effect
When monochromatic radiations of suitable frequency obtain from the source ‘S’, after being filtered by the glass filter, it falls on the photosensitive plate ‘C’, and emission of electrons called photoelectrons from ‘C’ which can get accelerated towards the plate ‘A’. The electrons flow in the outer circuit and hence the microammeter shows deflection. This is the working of the experimental study of the photoelectric effect.
Conclusion
It is to conclude that the photoelectric effect is defined as the phenomenon of an incident of light falling on the metal surface and the emission of electrons occurring. Einstein’s photoelectric effect is explained by an equation, that is K.E. max = h (ν – hν0); where, h = Plank’s Constant, ν = Incident photon’s frequency.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out