Phosphorous acids are considered as the oxyacids of phosphorous which do not have any color, there are other oxyacids of phosphorus acid as Hypophosphorous acid. The other name of phosphorous acid is orthophosphoric acid and the chemical formula is H3PO4. The density is 1.65 g/ cm3 and the boiling point is 200℃ and the melting point is 73.6℃ with a molecular weight of 82 g/ mol.
Phosphite anion is also known as phosphite salt. There are 2 anions of it which are Phosphinite and Phosphonite. The chemical formula for Phosphite Anion is HPO23–. They are referred to as inorganic in chemistry. It has a molecular weight of 78.9720 g mol-1.
USES OF PHOSPHOROUS ACID:
Uses of phosphorous acids are as follows:
It is a raw material in producing PVC stabilizers, amino methylene phosphoric acid, and hydroxy ethane di phosphoric acid.
It is used as a high power reducing agent.
It helps in producing raw materials of many items like fiber that are synthetic, phosphorous acid, organophosphorus pesticides, etc.
It is also used as the medium of producing high well-planned water treatment that saves and helps in using water efficiently.
USE OF PHOSPHITE SALT:
Uses of phosphite salt are:
It is used as a biodegradable fungicide that helps in curing plants of a disease that is very harmful to them which is Phytophthora Dieback. When applied, it automatically boosts the plant’s defense power which leads to the survival of the plant although being infected by Phytophthora dieback. This is the only treatment that can cure the plant of this disease, other than this there is no such treatment made or found.
Phosphites inorganic in nature are applied to the plants to fight against the molds or fungus that has been formed on the plant’s surface. Phosphite salts are used as fertilizers and sometimes they are formed into phosphates which are rich in nutrients that help the plant to grow.
The maximum part of the inorganic phosphates are used in the field of agriculture as monopotassium phosphite and they are used as fertilizers rich in potassium.
CHEMICAL AND PHYSICAL PROPERTIES OF PHOSPHOROUS ACID:
The physical properties of phosphorous acid are:
They have no color and the smell of acid is very bad or sour.
They can be seen in the form of white powder or can be called colorless.
They have 3 hydrogen bond acceptors
They have 1 covalent bond unit.
They are soluble in water so they have solubility.
The chemical properties of phosphorous acid are:
Phosphorous acids have very high power in reducing their properties and they can convert themselves into phosphoric acid. When it is heated at a high temperature then it breaks down itself into phosphine and phosphoric acid.
H3PO3 + 3H3PO3 = PH3 + 3H3PO4.
When phosphorous acid is put together with sodium hydroxide which is a base then it makes a reaction that leads to the formation of sodium phosphate and H2O which is water.
H3PO3 + 3NaOH = Na3PO3 + 3H2O.
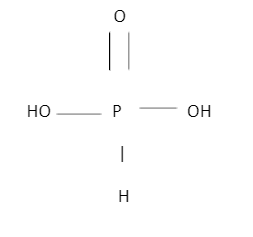
STRUCTURE OF PHOSPHOROUS ACID:
The structure of phosphorous acid looks like
![]() CONCLUSION:
CONCLUSION:
As we have come to know, Phosphorous acid and Phosphite Salt are very important as they are used in producing or manufacturing many raw materials which are used in producing many items like fertilizers, PVC stabilizers, etc. As phosphorous acid is a white powder-like substance so it does not have any color and has a bitter smell which is not liked Phosphite salts are used in plants so that the molds are the fungus that they are fighting with die and they should be free from the disease called Phytophthora dieback. Phosphorous acid can also be called hypophosphorous acid and orthophosphorous acid. Phosphite salt can break down into 2 items called phosphorite and phosphite.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out