The overlap of half-filled atomic orbitals (each holding a single electron) yields a pair of electrons shared between the two linked atoms, according to the Valence Bond Theory. When a portion of one orbital and a portion of a second orbital occupy the same region of space on two separate atoms, we say they overlap. A covalent bond is formed when two requirements are met:
(1) an orbital on one atom overlaps an orbital on another atom, and
(2) the isolated electrons in each orbital unite to create an electron pair.
The Molecular Orbital Theory was proposed to address the valence bond theory’s lack of comprehension of a molecule’s features. Multiple postulates define the generation of molecular orbitals from atomic orbitals and their attributes in the molecular orbital theory.
Electrons in an atom tend to repel one another, according to the Valence Shell Electron Pair Repulsion Theory. The atomic orbitals are stretched out and have larger bond angles to decrease this repulsion while building a molecule. The notion of ‘valence shell electron pair repulsion’ describes how orbitals are separated by large angles (VSEPR).
Types of overlapping
There are three types of overlapping:

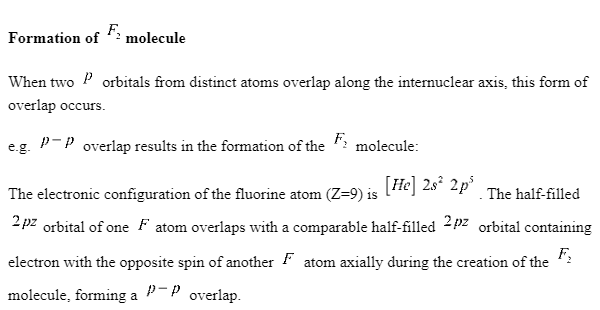
Conclusion
The overlap of two independent atomic orbitals on different atoms provides an area with one pair of electrons shared by the two atoms, according to the Valence Bond Theory. A bond is formed when the orbitals overlap along an axis that contains the nuclei. They establish a bond when they overlap in a way that generates a node along this axis.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out










