The Carius halogen technique is a quantitative method for determining halogens in chemical compounds in analytical chemistry.
In a furnaces, a fixed quantity of an organic compound is heated in the mere presence of silver nitrate placed in a hard glass tube called a carius tube. The compound’s carbon and hydrogen are oxidised to carbon dioxide and water. The presence of halogen results in the formation of silver halide (AgX). It is filtered, rinsed, dried, and weighed before being used.
This chemical test is as effective for determining sulphur levels without the use of silver nitrate. With the addition of barium chloride, the sulphuric acid intermediate generated by the interaction of sulphur with fuming nitric acid transforms into insoluble barium sulphate. The addition of nitric acid serves to oxidise carbon and hydrogen. Nitric acid in concentrated form solely oxidises iodine to iodic acid and has no effect on other halogens. Iodine oxidation by concentrated nitric acid occurs only at extremely high temperatures.
The Test of Lassaigne’s
It’s a test that looks for halogens, nitrogen, and sulphur in an organic molecule. The organic substances are covalently bound to these elements. These must be transformed into their ionic forms in order to be detected. The organic molecule is merged with sodium metal to attain this. The ionic chemicals formed during fusion are separated in aqueous solution and recognised using basic chemical testing. Sodium fusion extract, also known as Lassaigne’s extract, is the name of the extract.
Steps of Lassaigne’s test
(i) Lassaigne’s or sodium extract preparation: Sulphur in the organic constituent fuses with sodium to generate sodium sulphide.
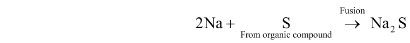
Sulphur analysis: The extract is separated into two components and sulphur analysis is performed as follows:
(i) Lead acetate test: A quantity of Lassaigne’s filtrate is acidified with acetic acid before being added to a lead acetate solution. The presence of sulphur in the organic component is confirmed by the formation of black precipitate.
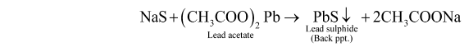
(ii) Sodium nitroprusside test: A few drops of sodium nitroprusside are added to another part of the Lassaigne’s filtrate. The presence of sulphur in the organic molecule is confirmed by the emergence of purple colour.
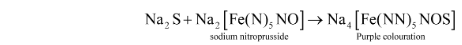
Estimation of Sulphur
Carius Method
Procedure
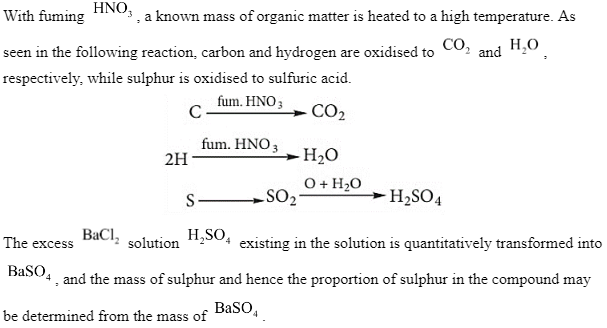
In a clean Carius tube, a known quantity of the organic compound is placed, and a few mL of fuming HNO3 is added. The tube is the one that is sealed. After that, it’s heated for around 5 hours in an iron tube. Allowing the tube to cool to room temperature, a tiny hole is drilled to allow the gases created inside to escape. The content of the Carius tube is collected in a beaker after it has been shattered. If there is too much in the beaker, the H2SO4 acid that forms as a result of the reaction is transformed to BaSO4 Filtered, cleaned, dried, and weighed BaSO4 precipitate. The proportion of S is calculated from the mass of BaSO4.
Calculations
Mass of the organic compound = w gram

= 36.78%
Conclusion
The Carius Method involves heating an organic compound in a sealed tube with silver nitrate in strong nitric acid to determine the quantity of sulphur and halogens present. Silver sulphide and halides are precipitated, separated, and weighed after the complex is decomposed. The Carius Method is used to calculate halogen.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out











