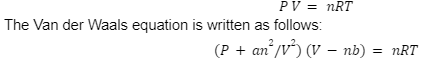A fundamental as well as important topic in chemistry, is that of the Van der Waals equation of state or more commonly just known as Van der Waals equation. The main question arises is what exactly is it and what is its application in the field of chemistry.
The Van der Waals equation deals with the effects of molecules which are interacting in gaseous state while extending this to the ideal gas law. The Van der Waals equation, in a way, can be called the extension of the ideal gas law. The derivation of the equation is also delved into detail.
What Is The Van der Waals Equation?
A fundamental as well as important topic in chemistry, is that of the Van der Waals equation of state or more commonly just known as Van der Waals equation. The main question arises is what exactly is it and what is its application in the field of chemistry.
The Van der Waals equation deals with the effects of molecules which are interacting in gaseous state while extending this to the ideal gas law. The Van der Waals equation, in a way, can be called the extension of the ideal gas law.
According to the ideal gas law, the relationship given below is that for ‘n’ number of moles that are occupied by any gas, in volume ‘V” and at pressure ‘P’ and temperature ‘T’ where the gas constant is denoted by ‘R’:
P V= nRT
The Van der Waals equation is written as follows:
(P+an²/V²) (V-nb)= nRT
So in this equation P, V, R, T, n stand for pressure, volume, gas constant, temperature and moles of gas respectively. The ‘a’ and ‘b’ stand for constants that are specific to a certain gas.
The units of the van der waals equation for a is atm lit2 mol-² and the unit for b is litre mol-¹.
Derivation Of The Van der Waals Equation
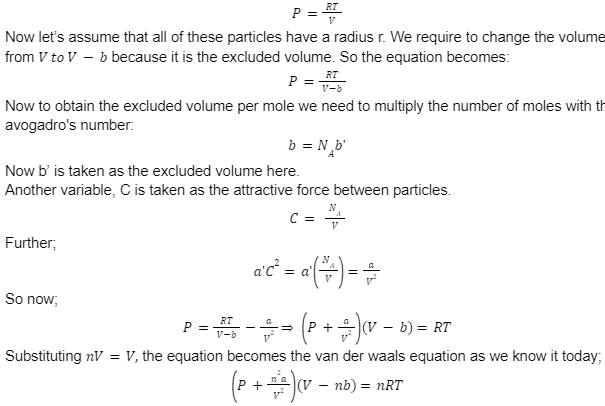
Let us now see how this equation was derived:
Conventional derivation:
Let us first consider one mole of gas that happens to satisfy the ideal gas law:
Applications Of The Van der Waals equation
This equation generally finds its use in calculating and figuring out how a gas might behave in the future. It is used to calculate and predict the behaviour of a gas under a non-ideal condition.
The Van der Waal equation cannot be used to predict and calculate properties of gas that are considered to be ideal. It finds its use only for non-ideal gases.
Conclusion
A fundamental as well as important topic in chemistry, is that of the Van der Waals equation of state or more commonly just known as Van der Waals equation. The main question arises is what exactly is it and what is its application in the field of chemistry.
The Van der Waals equation deals with the effects of molecules which are interacting in gaseous state while extending this to the ideal gas law. The Van der Waals equation, in a way, can be called the extension of the ideal gas law.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out