When electromagnetic radiation like light falls on a metal surface then the ejection of electrons from the metal surface occurs which is called the photoelectric effect. There is a photoelectric formula or equation by Einstein. The minimum frequency of the incident light that is required for the ejection of electrons for producing a photoelectric effect is known as the threshold frequency.
Photoelectric effect
The photoelectric effect is defined as the ejection of electrons from the metal surface when a wave of light that contains enough energy falls into a metal. The electron which gets ejected from the metal surface is known as photoelectrons because by the action of photons these photoelectrons are ejected. A question may arise in everyone’s mind that light of any energy can eject the photoelectrons from the metal surface? The answer is no because the work function of each metal is different, this work function is nothing but the minimum energy which is required by the electrons present on the surface of the metal to get ejected from its surface, so the quantum energy or the frequency of the photons must be either equal or greater than this particular work function to have a photoelectric effect.
Photoelectric effect formula
hν = W + E
Where,
ν = Incident photon’s frequency
E = The maximum kinetic energy that is ejected by the electrons = ½ mv²
W = Work function
h = Plank’s constant
Einstein’s photoelectric equation:
If a photon incident with a frequency is greater than the threshold frequency, the energy of a photon is given by
E = hν ……….. Equation (i)
h = Planck’s Constant
ν = Incident photon’s frequency
E = the ejected electron’s maximum K.E.
hν = φ0 + KEmax ………. Equation (ii)
φ0 = Work Function
If any photon is incident to threshold frequency then, hν0 = φ0
Φ0 = hν0 ………… Equation (iii)
From the Equation (ii) and (iii)
hν = hν0 + KEmax
- KEmax = hν – hνo
- KEmax = h(ν – ν0)
Threshold frequency
The threshold frequency is defined as, for producing a photoelectric effect, the minimum frequency of the incident light that is required for the ejection of an electron. The threshold frequency is denoted by γth. Such that we say hγth = φ
Where φ is the metal surface’s work function
So, if it is considered that γ is the frequency of the incident light and γth is the threshold frequency, then it can be assumed that:
- If γ = γth
So, from the metal surface the electrons i.e. the photoelectrons are ejected. Therefore, the electron’s kinetic energy is zero.
- If γ < γth
So, there will not be any kind of ejection of the photoelectron.
- If γ > γth
So, there will be the ejection of photoelectrons from the surface made by metal. The photoelectrons that are ejected from the metal surface contain some amount of kinetic energy.
Kinetic Energy can be represented as,
K.E. = ½ (mv²)
Stopping potential
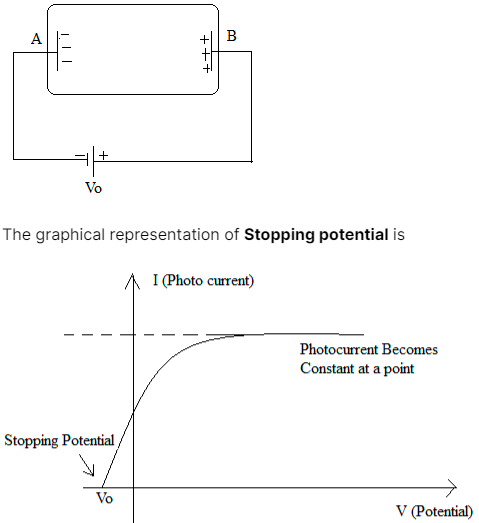
Stopping potential is the minimum negative potential given to an anode in a photocell for which the photoelectric current becomes zero. Stopping potential is the potential difference that is required to restrict the electrons from going towards the metal plate with a negative charge in the photoelectric effect.
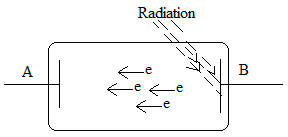
The above figure shows that when the radiation falls on plate B and electrons are ejecting from plate B and are travelling toward plate A. As we know, this diagram shows the photoelectric effect. If we add a variable voltage source (V0) between these two plates (given in the figure below) and create a negative charge on plate A. As it is known that electrons that are ejected from plate B are negatively charged electrons and now plate A also has negatively charged particles. Therefore, as a result of this electron will be rippled by the negative plate and after a certain voltage difference the repulsion between plate A and the electron will increase so much that a point reaches when no electron will have sufficient kinetic energy to move across the negatively charged plate A, So this voltage difference is known as stopping potential. Simply it can be said that the stopping potential should be at least equal to the K.E. of the electron so that the traveling of the electron towards plate A can be stopped. Therefore the value of V0 which makes the velocity of electron zero is termed as stopping potential. Stopping potential is denoted as V0.
Conclusion
It is concluded that the photoelectric effect is defined as the emission of electrons when incident light falls on the metallic material. Photoelectrons are the electrons that are ejected from the metal surface. The stopping potential is defined as the minimum negative potential given to an anode plate to stop the electrons from going towards the negative metal plate.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out











