In order to understand the nucleophile substitution reaction, we first need to understand the basics of chemistry. The basis of chemistry and physics revolves around the fact that like charges repel and opposite charges attract. In chemistry, we also learn that negatively charged electrons move from an area that is electronically rich to an area that is low on electrons. It’s due to this exchange of electrons that bonds are formed in a chemical reaction.
A nucleophile (nucleus loving) is an electron-rich species that shows a tendency to donate its free electrons to an electron-poor species or electrophiles, to form covalent bonds. Covalent bonds are a type of bond in chemistry in which pairs of electrons are shared between two atoms. Thus, when a neutrophile or an electron-rich species reacts with an electrophile or electron-deficient species, the reaction which occurs is an organic reaction. We shall get to know this with the help of an example further.
Now that we have understood what a nucleophilic reaction is, let’s understand the meaning of substitution reaction. As the word suggests, a substitution reaction deals with the replacement of an atom or group by another atom or group. In a nucleophilic substitution reaction, haloalkanes (alkanes with one or more halogen substituents) undergo a reaction in which a nucleus loving species displaces the halogen atom, which becomes a halide ion. This halogen ion, which is displaced from the central carbon atom of the molecule, is known as the leaving group. And this entire reaction involves nucleophile substitution. In alkyl halides, as the leaving group is a halide ion, it is relatively stable and thus makes good leaving groups.
Thus in order to understand a nucleophilic substitution reaction in detail, let us take the example of the following substitution reaction:
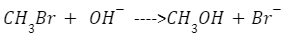
Here, methyl bromide is an electrophile, and OH- is the nucleophile. Thus, the Br or Bromine in the reactant is replaced by the nucleophile, and thus we get methanol as the major product and Br- as the leaving group. In simpler terms, the free electron pair present in the nucleophile attacks the substrate, while this C-X bond of the substrate undergoes heterolysis and this lone pair of electrons bonds with it. At the same time, the leaving group from this reaction departs with the electron pair.
Thus, we learned that a nucleophilic substitution reaction is one where a nucleophile that is electronically rich attacks the positively charged electrophile, thus replacing the leaving group.
The extent to which a species can accept or donate a pair of electrons is called its electrophilicity or nucleophilicity. So, the extent to which a species would be able to accept a pair of lone electrons is called its electrophilicity. The extent to which a species will be able to donate electrons is called its nucleophilicity. And this, in turn, will determine its reactivity.
In 1935, two scientists studied nucleophilic substitution reactions concerned with alkyl halides and their related compounds. They proposed that there were two primary mechanisms that competed with each other, otherwise known as SN1 and SN2 reactions. Where N stands for nucleophilic and S for substitution, while 1 and 2 state the reaction’s kinetic order, studying the kinetics of a chemical reaction helps us understand how fast the reaction could occur. SN1 reactions are unimolecular nucleophilic substitution reactions or first-order reactions, while SN2 are bimolecular nucleophilic substitution reactions or second-order reactions.
Conclusion
Thus, to conclude, a nucleophilic reaction or nucleophilic substitution reaction is a chemical reaction happening between nucleophile and electrophile, where the electron-rich species replaces the functional group of an electrophile, and the molecule which leaves this electrophile with the pair of electrons is termed as leaving group. Nucleophilic reactions are a common type of chemical reaction seen in organic chemistry.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out











