The Ideal Gas Law is based on the idea that gases are made up of point masses that crash in a totally elastic manner. At low temperatures or high tensions, notwithstanding, real gasses depart from these standards. Think about a container with increased pressure. The volume of the container decreases as the tension ascents.
The volume filled by the gas particles is as of now not insignificant in comparison to the volume of the container, and it should be taken into account. The active energy of gas particles is smaller at low temperatures; subsequently they move more slowly. The intermolecular powers acting on the gas particles impact them, bringing about inelastic crashes between them. Thus, there are less crashes with the container and the tension is lower than what might be anticipated from an ideal gas.
The Ideal Equation:
An ideal gas is a hypothetical gaseous material whose behavior is impeccably characterized by the ideal gas law and is free of attracting and shocking powers. There is no such thing as an ideal gas in reality, yet it is a valuable conceptual model for understanding how gasses react to changing conditions.
As we will see, most real gasses display behavior that nearly looks like that of an ideal gas under a variety of situations. Under most circumstances, the ideal gas law can be utilized to foresee the behavior of real gasses. At extremely low temperatures or exceptionally high tensions, when deviations from ideal behavior are most normal, the ideal gas law doesn’t perform well.
Coming up next is the Ideal Gas Equation:
P V = nRT
Four unique qualities of a gas are addressed by the four variables:
Pressure (P) is a unit of measurement that is ordinarily communicated in atmospheres (atm), kilopascals (kPa), or millimeters mercury/torr (mm Hg, torr)
Volume (V) is measured in liters.
The quantity of gas moles (n)
The gas’ temperature (T) is measured in degrees Kelvin (K)
R stands for the ideal gas constant, which takes many structures relying upon the units utilized. The three most popular R formulations are as per the following:
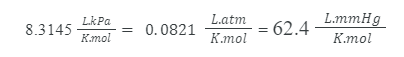
An Ideal Gas Examples:
Example 1:
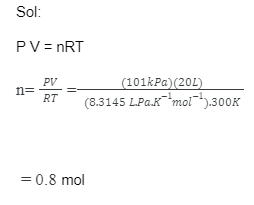
A limited amount of gas is contained in a 20 L box at a temperature of 300 K and a strain of 101 kPa. In the crate, what number of moles of gas are there?
Example 2:
Decide the amount of moles of gas contained in a 20.63 cubic meter jumping castle with a temperature of 300 Kelvin and a tension of 101 kPa.
Sol:
P V = nRT

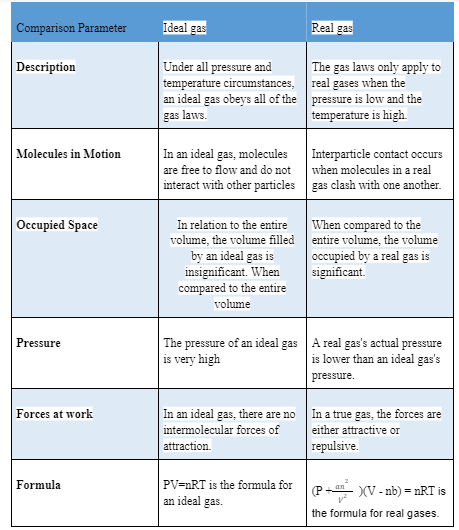
The difference between a Real and an Ideal gas:
CONCLUSION:
The Ideal gas is made up of many particles that travel randomly every which way and is liberated from interparticle interaction. It adheres to both the gas laws and the equation of state. Impacts between particles in an ideal gas are completely elastic, and that means that no active energy is lost when a crash happens.
There are no intermolecular powers of attraction in an ideal gas. It’s an imaginary gas, which suggests it doesn’t exist in reality. In both Newtonian and quantum physical science, the model of an ideal gas law has been investigated.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out











