Johan G.C.T. Kjeldahl, a Danish scientist, invented this technique in 1883 to quantify the nitrogen concentration in organic and inorganic compounds (like foodstuffs, fertilizers, wastewater, soil, feed, grain, and other substances). This approach is also used to figure out how much protein is in a certain meal.
The Kjeldahl method is an analytical chemistry technique that aids in the quantitative measurement and quantification of nitrogen in both organic and inorganic molecules. Johan Kjeldahl was the first to create the approach in 1883. This method of protein analysis relies heavily on this process. This approach was created to figure out how much nitrogen is in organic and inorganic compounds (like foodstuffs, fertilizers, wastewater, soil, feed, grain, and other substances).
Equipment and Apparatus
Stone fume hoods and gas mantles were used to conduct the Kjeldahl procedure at first. Macro-Kjeldahl digestion, as well as a distillation device, were created and employed a few years later. Kjeldahl flasks were also used in the arrangement. Micro-Kjeldahl equipment, which comprises of smaller sized pieces, is a tiny version of the setup.
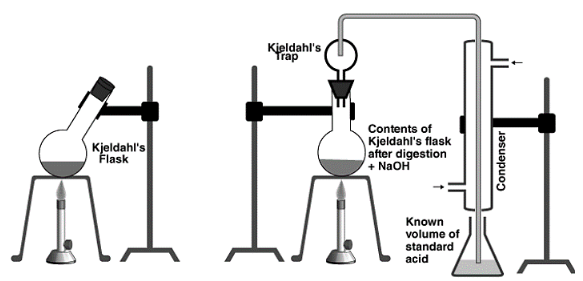
In recent years, the equipment has undergone major alterations, including the use of aluminium or ceramic heating blocks. This design can also accommodate many straight digestive tubes at the same time. Furthermore, “Block digesters” are utilised in conjunction with tabletop distillation machines with steam generators to reduce distillation time. The majority of the equipment is composed of corrosion-resistant materials.
The Kjeldahl Method entails a procedure
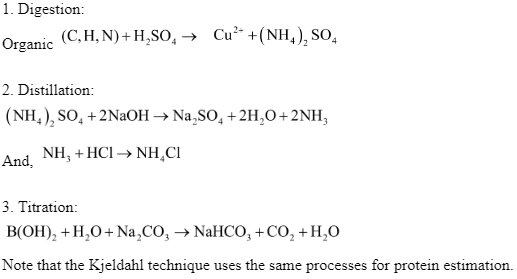
The three-step procedure detailed below is the working principle of Kjeldahl analysis:
Digestion
The given or taken organic sample is first treated with a strong acid solution, usually H2SO4 . At a very high temperature, the solution is boiled. The material is digested by the acid solution, which produces ammonium sulphate solution.
Distillation
A mixture of boiling and condensation is used in this technique. To convert the ammonium sulphate solution to NH3 gas, an excess of base is added to the produced solution.
Titration
The produced product from the preceding step is titrated to yield the final requisite results in order to measure the nitrogen contained in the sample.
This strategy had a significant influence. Furthermore, research have increased the method’s quality. Various scientific organisations have deemed this procedure to be one of the most adaptable. AOAC (Association of Official Analytical Chemists), AACC (Association of American Cereal Chemists), AOCS (American Oil Chemist Society), EPA (Environmental Protection Agency), ISO (International Organization for Standardization) are some of these organisations (International Standards Organization). The names of some of the organisations that lead the scientific equipment for conducting the Kjeldahl technique, also known as VELP.
Kjeldahl Method Reaction:
Kjeldahl Method Formula
NH3 has a weight equivalent of 17g/eq. In addition, one equivalent weight of NH3 contains 14 grams of nitrogen. As a result, the proportion of nitrogen may be calculated using the formula:
Nitrogen quantification by the Kjeldahl technique = 1.4V NW
Where W is the weight of the sample that was utilised (in grams), V is the titration acid (in ml), and N is the standard acid’s normality.
Kjeldahl Method Limitations
Only nitrogen attached to organic components (proteins, amino acids, nucleic acids) and ammonium in the sample are measured using this approach. This approach is ineffective for compounds with nitrogen in the azo and nitro groups, as well as in rings (quinoline, pyridine, nitrate, and nitrite, etc). The nitrogen in these compounds can’t be transformed to ammonium sulphate using the Kjeldahl technique.
Conclusion
The Kjeldahl technique was invented in 1883 by a Danish chemist called Johan Kjeldahl. This method was created particularly for measuring the nitrogen content of organic and inorganic materials.
Kjeldahl nitrogen determinations are employed on a variety of samples nowadays, including wastewater, soil, fertilisers, meat, feed, grain, and a variety of other substances. The approach is also used to determine the amount of protein in foods.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out











