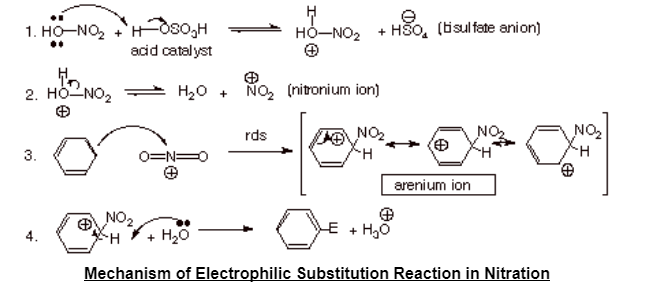
Electrophilic Substitution Mechanism in Nitration is the mechanism of electrophilic substitution reaction in which the nitronium ion (NO3+), an electrophile form the nitric acid attacks on the benzene ring which further will finally form nitrobenzene.
Substitution reaction is the reaction in which one functional group substitutes the another one in a chemical compound and can be done in both aliphatic and aromatic reactions by the help of either electrophile (electrophilic substitution reaction) or nucleophile (nucleophilic substitution reaction).
Electrophilic Substitution Reaction is the reaction in which a functional group like ketone, ether, amines etc. is displaced by an electrophile. Both the aliphatic as well as aromatic compounds undergo substitution reaction or electrophilic substitution reaction. There are two types of electrophilic substitution reaction;
- Electrophilic Aliphatic Substitution Reaction: is the reaction in which electrophile attacks on the functional group of the compound of aliphatic reaction.
- Electrophilic Aromatic Substitution Reaction: is the reaction in which the electrophile attacks on the aromatic benzene ring in the reaction.
There are 3 stages for the electrophilic aromatic substitution reaction are as follows;
- Activation of the electrophile (it is the step or stage in which the electrophile like Cl, Br etc. becomes activated or generates in the presence of Lewis acid).
- Electrophile attacking on the aromatic benzene ring.
- Finally deprotonation happens.
Mechanism of Electrophilic Substitution Reaction of Nitration on Benzene Ring
Electrophilic Substitution Reaction of Nitration on Benzene ring occurs when benzene will react with conc. HNO3 (concentrated nitric acid) at temperature of 322 to 333 Kelvin for the formation of nitrobenzene in the presence of conc. H2SO4 (concentrated sulphuric acid). It is basically the process in which the Nitro group gets attached to the aromatic benzene ring.
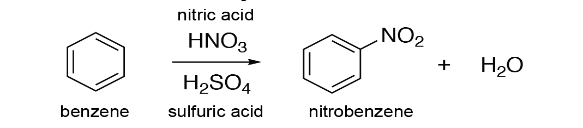
It is done in 3 steps;
Step 1: Activation of an electrophile or Electrophile generation
Nitronium ion (NO3+) is formed by dissociation when a proton is accepted by the concentrated nitric acid from conc. H2SO4 (sulphuric acid).
Step 2: Electrophile attacking on the aromatic benzene ring
An electrophile nitronium ion reacts with the aromatic ring benzene for the formation of arenium ions.
Step 3: Deprotonation
Finally the nitrobenzene compound is formed when the arenium ion formed in the above step loses it’s proton to the Lewis Base.
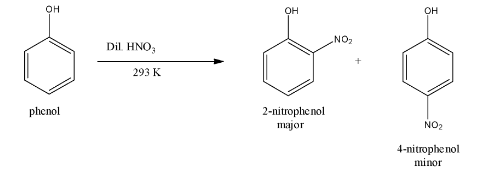
Mechanism of Electrophilic Substitution Reaction of Nitration on the Phenol
Phenol is the aromatic compound in which the hydroxyl group (OH) is attached to the benzene ring at ortho position mainly. Electrophilic Substitution Reaction of Nitration on the Phenol is the reaction or mechanism in which the substitution happens on the Phenol ring i.e. the electrophile attacks on the Phenol ring to form nitrophenol.
Phenols undergo nitration when treated with dil. HNO3 (dilute nitric acid) at 293-298 Kelvin (low temperature) and then they form the combination of ortho nitrophenol (2-nitrophenol) as the major product and para nitrophenol (4-nitrophenol) as the minor product which will be separated by the process of steam distillation depending on their volatility. Para nitrophenols are more volatile than the ortho nitrophenol due to the hydrogen bonding intermolecularly.
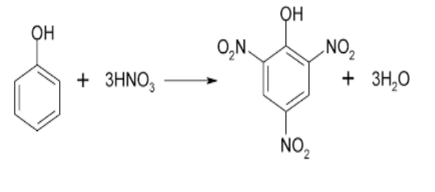
2,4,6-trinitrophenol is formed when the aromatic compound phenol undergoes nitration and reacts with conc. HNO3 (concentrated nitric acid). The compound 2,4,6-trinitrophenol is commonly known as picric acid. This is depicted by the following picture given below;
Conclusion
The substitution reaction is the reaction which substitutes the functional group with another and electrophilic substitution reaction is the reaction in which the electrophile like Cl, NO2, and many more replaces or substitutes the other group of aliphatic or aromatic compounds. The electrophilic substitution mechanism in nitration is the mechanism by which the electrophilic substitution reaction occurs with the help of generation of an electrophile.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out











