Chemistry has many different reactions to different compounds, making it important for the students to understand all about them. The Sandmeyer and Gattermann reactions are examples of exclusive reactions that have a special place in their applications. Hence, it becomes important for students to learn all about the Sandmeyer reaction definition and Gattermann reaction definition.
Let us help our students understand all about these two reactions and their common examples. We’ll cover the main differences between the Sandmeyer and the Gattermann reactions in detail. Let us start with the quick definitions of both reactions.
Sandmeyer reaction definition:
In this reaction, aryl halides are synthesised from the aryl diazonium salts. Copper is used as a catalyst in the Sandmeyer reaction. All these reactions belong to radical-nucleophilic aromatic substitution reactions—any processes like hydroxylation, trifluoromethylation, cyanation, halogenations, etc., of benzene.
Sandmeyer reaction example:
A simple example of Sandmeyer’s reaction is:
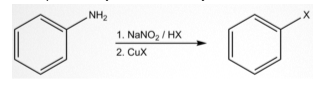
In this reaction, the nitrous acid is prepared in the process from acid and sodium nitrite. One part of the water is lost to create a nitrosonium ion, which acts as an electrophile in the Sandmeyer reaction. This nitrosonium ion reacts with the aromatic amine to form a diazonium salt. This reaction offers different functions on the benzene ring. These processes are achieved as follows:
- Chlorination using CuCl
- Bromination using CuBr
- Cyanation using CuCN
- Hydroxylation using Cu2O
- Fluorination using tetrafluoroborate anions
Gattermann reaction definition
In this reaction, aromatic compounds are formylated. The catalysts used in the Gattermann reactions are Lewis acids. The formulation process is achieved using a mixture of hydrochloric acid (HCl) or hydrogen cyanide (HCN). The commonly used Lewis acid is AlCl3. Further, HCl or HCN can be replaced with zinc cyanide.
Gattermann reaction example:
A simple example of Gattermann reaction is:
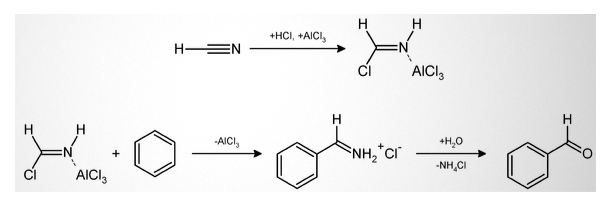
It is easy to replace hydrogen cyanide with sodium cyanide or cyanogen bromide. The combination of zinc cyanide can be used in the reaction. While zinc cyanide is highly toxic, its solid form makes it easy to work in the Gattermann reactions having gaseous HCN. The zinc cyanide reacts with HCl to form the main HCN reactant and zinc chloride in this process. It further acts as an in-process Lewis acid catalyst.
Difference between Sandmeyer reaction and the Gattermann reaction
Both the Sandmeyer reaction and the Gattermann reaction are organic reactions. However, these reactions are different when it comes to the participating reagents, catalysts, and final products. Both reactions have different use cases. Let us understand the main differences between the Sandmeyer reaction and the Gattermann reaction one-by-one:
Sr. No. | Topic | Sandmeyer reaction | Gattermann reaction |
1 | Definition | It is the reaction in which aryl halides are synthesized aryl diazonium salts. It is the type of organic reaction substitution. | It is the reaction in which formylated aromatic compounds are formed. It is also a type of organic reaction substitution. |
2 | Involving reactants | Aryl diazonium salts | Any aromatic compounds |
3 | Type of catalysts | Copper salts | Lewis acids |
4 | Final products | Aryl halides | Formylated aromatic compounds |
5 | Uses | It is used in hydroxylation, trifluoromethylation, cyanation, halogenation, etc., of benzene. | It is used in introducing the aldehyde groups to the benzene ring. |
Conclusion
Hence, it is easy to understand the difference between the Sandmeyer reaction and the Gattermann reaction. Starting with the definitions of both reactions, it is easy to understand the difference in the processes. The key differences between the Sandmeyer and the Gattermann reactions make it easy for students to learn the different applications.
The quick list of the frequently asked questions makes it easy to answer the common queries and questions related to these reactions.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out













