Radiation does not require any kind of material medium to transport heat. A body emits energy, which travels through space in the same way as light does. When it collides with a material body, a portion of it is absorbed, and the receiving body’s thermal energy is enhanced. This is how the sun’s heat reaches the planet, after travelling millions of kilometres over empty space.
Since the radiation released by bodies as a result of their temperature falls almost entirely within this range, thermal radiation is also described as the component of the electromagnetic
Thermal Radiation
Thermal radiation released as a result of energy changes of molecules, atoms, and electrons of a substance is the sort of electromagnetic radiation that is relevant to heat transmission. The rate of thermal radiation emission increases with rising temperature, and temperature is a measure of the strength of these processes at the microscopic level. All objects with a temperature greater than absolute zero emit thermal radiation continually. That is, everything in our environment, including walls, furniture, and our friends, emits (and absorbs) radiation at all times.
Radiant energy can be absorbed, transmitted, or reflected when it strikes a surface. The total energy emitted is equal to the sum of these three effects, and the parameters that describe these three phenomena are provided by

contains the radiation emitted by things at room temperature. At temperatures exceeding 800 K, bodies begin to emit visible radiation. A lightbulb’s tungsten filament must be heated to temperatures exceeding 2000 K before it can generate any meaningful amount of visible radiation.
- Ultraviolet radiation: Between the wavelengths 0.01 to 0.40 μm ultraviolet light comprises the low-wavelength end of the thermal radiation spectrum. Ultraviolet rays should be avoided since they can kill microorganisms and harm people and other living creatures. The UV range makes up about 12% of solar radiation, and it would be disastrous if it reached the earth’s surface. Although, the ozone (O3) a layer in the atmosphere works as a shield, absorbing the majority of the UV rays.
Effects of radiation
Radiation is everywhere in our surroundings and it can be used safely in a variety of purposes. Depending on the dose, the consequences of radiation might be moderate or fatal. Nuclear accidents, the workplace, and some medical treatment are all possible sources of radiation poisoning. There is no cure, although barriers can be used to prevent exposure and some drugs can help to remove some of the radiation from the body. Anyone who suspects they are radiation-sensitive should see a doctor right away.
Greenhouse Effect
Over 90% of solar radiation is emitted through a transparent window in the wavelength glass at room temperature to the range. The total radiation emitted by surfaces at normal temperature, on the other hand, falls in the infrared zone. As a result, glass allows solar radiation to pass through but prevents infrared radiation from the inner surfaces from escaping.
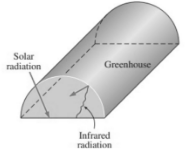
As a result of the energy build-up, the interior temperature rises. Because it is used largely in greenhouses, this heating effect is known as the greenhouse effect. It is caused by the nongray feature of glass (or clear polymers).
Examples of radiation
All radioactive materials, on the other hand, release radiation. The following are some instances of several forms of radiation: x-rays from x-ray equipment, ultraviolet light from the sun heat from a stove burner visible light from a candle, sound waves from your stereo microwaves from a microwave oven, electromagnetic radiation from your cell phone, alpha particles emitted by the radioactive disintegration of uranium, a black lamp emits ultraviolet light, etc.
Applications
- Medical Application: The use of x-rays, a sort of radiation that may pass through human skin, is the most prevalent of these medical treatments. Because our bones and other structures are denser than our skin, they produce shadows when x-rayed, and those shadows may be seen on photographic film. Placing a pencil under a piece of paper and holding the pencil and paper in front of light produces a similar effect.
- Academic and Scientific Applications: We’ve learned more about the types of soil that different plants require, the magnitude of newly discovered oil fields, and the paths of ocean currents thanks to radiation. Carbon dating is a method used by archaeologists to establish the age of fossils and other items using radioactive chemicals.
Conclusion
The emission and propagation of energy are referred to as radiation. Because radiation comprises all types of energy, a substance does not need to be radioactive to emit radiation. The effects, types, and applications explained above give full insight into radiation in our environment.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out











