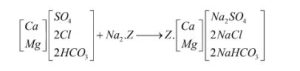Introduction:
In living creatures’ biological systems, ion-exchange reactions are crucial. They are vital not only in the selective secretion of specific biological substances but also in the transport mechanism of certain ions across cell membranes, as well as in nerve signal expansions.
Ion exchange mechanisms are important in agriculture as well because the ion-exchange characteristics of natural silicates in soils have a significant impact on the composition of the interstitial liquid available for plant feeding.
Ion exchange:
Any of a series of chemical reactions involving the exchange of one or more ionic components between two substances, each consisting of positively and negatively charged entities termed ions exchange reaction. Both the contaminant and the material to be exchanged must be dissolved and have the same electrical charge type (positive or negative).
The process of “water softening,” which aims to reduce calcium and magnesium concentration, is a good example of ion exchange. Ion exchange, on the other hand, is effective at removing hazardous metals from water.
Ion Exchangers:
Depending on their chemical structure, ion exchangers can be unselective or have binding preferences for specific ions or classes of ions. This can be influenced by the size, charge, or structure of the ions. are some examples of ions that can bind to ion exchangers.
The favorability of any particular ion and the number of active sites available for this exchange are two essential elements that affect the efficiency of an ion exchange resin. Because resin contains active groups in the form of electrically charged sites, large surface areas are often preferred to maximize active sites.
The following are examples of ion exchangers:
• Positively charged ions are exchanged by cation exchangers (cations)
• Negatively charged ions are exchanged by anion exchangers (anions)
• Amphoteric Exchangers: These exchangers exchange both cations and anions at the same time.
As pure water is often required for the effective development of a product and the prevention of corrosion in industrial applications.
Ion-exchange Process:
A microporous exchange resin must be present in the equipment for the ion exchange process to take place. This resin is soaked with a very loosely held solution. Sulfonated polystyrene beds are placed within the unit when the technique is utilized for water softening. The sodium solution that covers the surface of these beds is saturated. The ions bond directly to the beads when water passes through the resin bed, releasing the aforementioned solution into the sample water.
The exchange resin will need to be recharged or regenerated when the beds get saturated with pollutants over time. It is critical to flush the exchange resin with a salt brine solution to finish the regeneration process. Sodium ions make up the salt brine solution. The impurities that coat the resin bed will be replaced by these ions, which will then be washed away with the wastewater. Because of the way the ion exchange process works, it may soften hard water with high levels of magnesium and calcium while simultaneously being utilized for water treatment.
Sodium zeolite Water softening:
The most common application of ion exchange is sodium zeolite softening. Water containing scale-forming ions, such as calcium and magnesium, travels through a resin bed containing sodium-form SAC resin in zeolite softening. The hardness ions exchange places with sodium in the resin, and the sodium diffuses into the bulk water solution. The soft water, which is devoid of hardness, can subsequently be utilized for low to medium pressure boiler feedwater, reverse osmosis system makeup, some chemical operations, and commercial uses like laundries.
The following reaction describes how a zeolite softening process removes hardness from water:
Hardness is almost non-existent in water from a well-run zeolite softener. However, minor levels of hardness, referred to as leakage, can be found in the treated water. Hardness leakage is determined by the hardness and sodium content of the influent water, as well as the amount of salt needed for regeneration.
Ion-exchange in Zeolite:
The cations that make up a zeolite framework are mobile and can be swapped out for others. A negatively charged blue zeolite (aluminosilicate) framework is depicted in the diagram below. Positively charged ions (red) fill the holes, balancing the negative charges. Other cations can be substituted for the red cations (yellow).
Zeolites and other porous crystalline structures can exchange ions with radioactive ions in order to clean up nuclear waste using zeolites and other cage-like materials. Zeolites aren’t merely ion trappers. They’re also found in pet litter, for instance. The zeolites’ porous crystalline structure aids in the trapping of undesirable liquids and smells compounds.
Other Applications:
In the laboratory, ion exchange is utilized for both analytical and preparative purposes, with analytical applications being the most common. This is a common use of ion-exchange chromatography. In medicine, ion-exchange resins have a restricted application. By ingestion, carboxylic resins containing hydrogen or ammonium ions remove sodium ions from the gastrointestinal tract, reducing edema.
Conclusion:
Various charged atoms or molecules (ions) such as nitrates, fluoride, sulfates, perchlorate, iron, and manganese ions, as well as hazardous metals (radium, uranium, chromium, etc.) can be removed from water via ion exchange methods. Ion exchange is most commonly used in the chemical sector to prepare high purity water for industrial purposes, water softening, and metal recovery or removal.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out