Before getting into the main topic, let’s get a basic idea of the concept so,
There are four types of orbitals to master: s, p, d and f (sharpness, goal, distribution and basic). Inside each of the atomic shells are certain compounds of orbitals. In the shell n = 1, you get only s orbitals. In the n = 2 shell, you have orbitals us and p; in the n = 3 shell, you have s, p and d orbitals, and in n = 4 up shells, find all four orbital species. It is important to note here that these are orbitals, shells etc. they are all part of an empirical theory designed to explain what we see in relation to cell structure and fusion.
There is just one orbital electron in each n-orbital n. Because its angular quantum number (l) is zero, it has a zero quantum magnetic number. It is possible to have either an electron spin up (ms = 1/2) or spin down (ms = -1/2). If there are two electrons, they must spin up and down at the same time.
S orbital
The orbital shape is spherical; round orbitals are round. The n orbital nodes are n-1; angular nodes are l, which is 0 of all orbitals; radial nodes are n-l-1, which is n-1 for all orbitals. Therefore, the orbital has only radial nodes, which are a sphere.
When n increases, orbitals become larger, extending away from the nucleus. They contain additional nodes. This is similar to a standing wave with significant amplitude regions separated by nodes, points with zero amplitude. In a given atom, orbitals also have a higher potential as n increases due to their increasing distance from the nucleus.
The shape of an s orbital
An s orbital is spherically symmetric around the nucleus of the atom, a sort of a hollow ball made from rather fluffy material with the nucleus at its centre. Because the energy levels increase, the electrons are located beyond the nucleus. Therefore the orbitals get bigger. The order of size is 1s < 2s < 3s <…,
Now, let’s check out a cross-section of those orbitals.
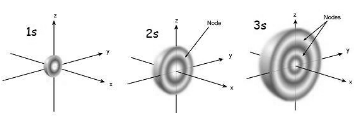
When looking at the above diagram, you’ll note that a 1s orbital has little or no electron density near the nucleus, but it grows up to a maximum outside the nucleus and then begins to fall. It looks like a hollow ball. Unlike a 1s orbital, a 2s orbital has an electron density sphere inside an outer electron density sphere, like one ball inside another. There is an area between the two balls where there is no chance of detecting an electron. This surface is referred to as a node or a nodal surface. A 3s orbital contains three nodes, making it considerably bigger than a 2s orbital.
In all directions, such as orbitals (the top line in the animation image below), it can be seen that the centre of the drum is very vibrant, with an antinode in all atomic orbitals. This antinode means that the electron may be in the visible position of the nucleus (passing straight through without breaking it or hitting it), as it travels (on average) much faster at that moment, giving you greater momentum.
Electron filled in the s orbital
The electron can have an orbital itself, but it would like to take a low-energy orbital with another electron before taking a high-energy orbital. In other words, within a single energy level, electrons will fill the orbital before it begins to replenish.
A subshell can hold 2 electrons.
What happens to s orbitals when n increases?
- They become larger, extending far beyond the nucleus.
- They contain additional nodes. This is similar to a standing wave with significant amplitude regions separated by nodes, points with zero amplitude.
- In a given atom, orbitals also have a higher potential as n increases due to their increasing distance from the nucleus.
Conclusion
We know what s orbital is and also discussed its shape orientation and other parameters. As of now, with this, you can build a basic idea about s orbital and will be able to understand further complexities of this concept easily.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out











