The Kjeldahl method is an analytical chemistry technique that aids in the quantitative measurement and quantification of nitrogen in both organic and inorganic molecules. Johan Kjeldahl was the first to create the approach in 1883. This method of protein analysis relies heavily on this process. This approach was created to figure out how much nitrogen is in organic and inorganic compounds (like foodstuffs, fertilisers, wastewater, soil, feed, grain, and other substances).
This approach is based on the fact that free nitrogen is produced when nitrogenous compounds are burned with cupric oxide in a CO2 environment. Thus By travelling over a heated copper spiral, traces of nitrogen oxide, which can occur in some instances, are converted to elemental nitrogen.
The CO2 generator, combustion tube, and Schiff’s nitrometer are used in the Dumas technique.
Kjeldahl method
It is widely used to determine the amount of nitrogen in fertilisers, foods, pharmaceuticals, and other products. However, the approach is ineffective for compounds with nitrogen in the ring (e.g., pyridine, quinoline, etc.) and nitrogen directly connected to an oxygen atom (e.g., NO) or another nitrogen atom (e.g., azo compounds).
The device was used to calculate nitrogen using Kjeldahl’s method.
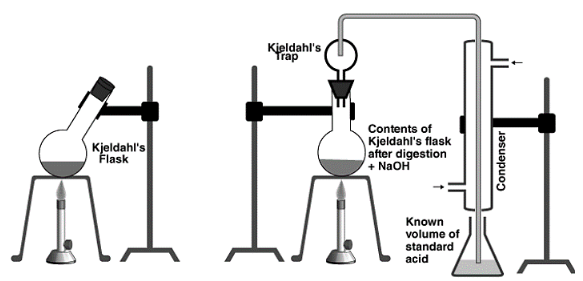
Principle: In a long-necked flask known as a Kjeldahl’s flask, a known mass of the organic compound is heated with conc. H2SO4 in the presence of potassium sulphate and a little copper sulphate or mercury. While copper sulphate or mercury catalyse the reaction, potassium sulphate elevates the boiling point of H2SO4, ensuring full reaction.
The nitrogen in the organic component is quantitatively transformed to ammonium sulphate as a result of heating. The resulting ammonium sulphate is cooked in an excess of NaOH solution to liberate ammonia gas, which is then absorbed in a known excess of a standard acid such as H2SO4, or HCI.
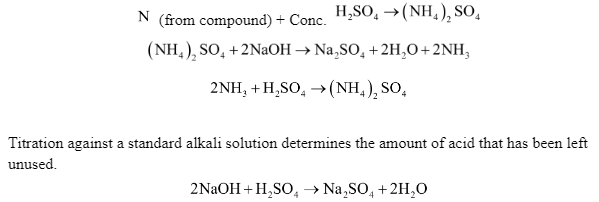
The volume of acid used (or the volume of ammonia produced) and hence the proportion of nitrogen in the organic molecule may be estimated using this information.
Dumas Method
This approach may be used on any organic substance that contains nitrogen.

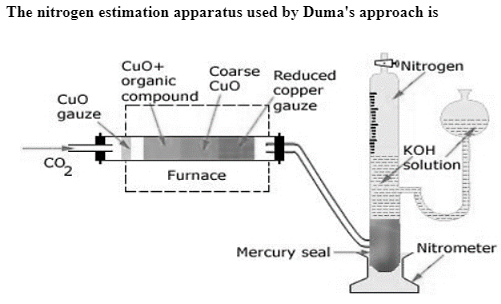
CO2 generator: CO2 is produced in this procedure by heating magnetite or sodium bicarbonate in a hard glass tube, or by dilution HCI on marble in a kipps apparatus. After being dried by bubbling through Conc. H2SO4 . The gas is sent via the combustion tube.
Combustion Tube: The combustion tube is charged with a) A roll of oxidised copper gauze to prevent back diffusion of combustion products and to heat the organic substance combined with CuO via radiation) a weighed amount of the organic substance mixed with excess CuO , C) a layer of course CuO packed in about 2/3 of the length of the tube and held in place by a loose asbestos plug on either side; this oxidises the organic vapours passing through it, and d) a reduced copper spiral that converts any nitrogen oxides formed during combustion to nitrogen.
Schiff’s nitrometer: Nitrogen gas is produced by breakdown of a chemical in a combustion tube, and it is combined with a large amount of CO2. When CO2 is absorbed by KOH and the nitrogen is collected in the top half of the graduated tube, it is quantified using a passing nitrometer.
Conclusion
Kjeldahl nitrogen determinations are employed on a variety of samples nowadays, including wastewater, soil, fertilisers, meat, feed, grain, and a variety of other substances. The approach is also used to determine the amount of protein in foods.
Historically, the Dumas technique of molecular weight measurement was employed to determine the molecular weight of an unknown material. The Dumas technique is suitable for determining the molecular weights of volatile organic liquids at room temperature.
The approach is named after the French scientist Jean Baptiste André Dumas, who devised the method. The approach was used by Dumas to calculate the vapour densities of elements (mercury, phosphorus, and sulphur) as well as inorganic compounds.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out













