The tetrahedral shape is formed when four atoms in their elemental form join together by covalent bonding. By the word “tetrahedral,” we can get a slight idea about the meaning of this term. “Tetra” means the number four, and the word “hedral” depicts a solid face. When we combine the meaning of both these terms, we know that tetrahedral means a solid with four faces. Molecular geometry is the name of the study that is done on different atoms that combine with one primary atom at the center by bonding and forming a specific physical structure. The molecular structure of a three-dimensional molecule doesn’t change rapidly and is found in nature in the same orientations. The tetrahedral geometry is widely seen in the molecules, and it also has different bond angles. In this article, you’ll learn what is the tetrahedral shape of a molecule and what are some compounds that exist in the tetrahedral shape.
What Is Tetrahedral Geometry?
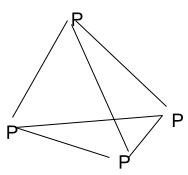
A tetrahedron is a three-dimensional closed shape made up of four faces. Each face of a tetrahedron is an isosceles triangle. A tetrahedron is also called a pyramid with a triangular base. There are four vertices and six edges in a tetrahedron.
A tetrahedron is actually resembling solid by the help of which we can easily understand what is a tetrahedral geometry and its properties.
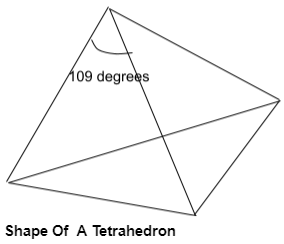
The tetrahedral molecule has four single covalent bonds therefore, the hybridization is sp3. The angle of the pyramid is 109 degrees, and the bond angles at the bottom triangles are less than 109 degrees i.e., 107 degrees.
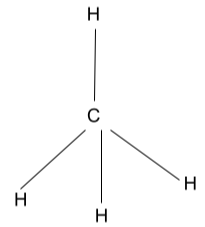
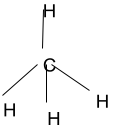
The most commonly formed molecule on the earth, methane (CH4), is a covalent compound. It is also found in the tetrahedral structure, with carbon being the central atom because it has a covalency of four and shares it with four hydrogen atoms. Methane is also a three-dimensional molecule.
Variations In Tetrahedral Geometry
The tetrahedral shape of molecules can be found in many different variants:
- When there is zero lone pair: – This three-dimensional molecule is formed when the four valence electrons of the central atom get distributed among four other acceptor atoms, thereby forming four single covalent bonds.
All four bonds are not in the same plane. One single bond will be emerging out of the screen plane, one bond will be entering inside the screen plane, and two single bonds atoms are present in the screen plane.
When a tetrahedral shape is formed by the central atom with no lone pair, the chemical hybridization is sp3. The bond angle associated with this variant is 109.6 degrees. The obvious example is CH4.
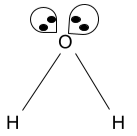
- When there is some lone pair: – This three-dimensional molecule is formed when the four valence electrons of the central atom get distributed to two other acceptor atoms, and the remaining electrons are contributing to the formation of lone pair.
This variant comes along with AX2E2 (A=central atom, X= electron acceptor, and E = lone pair) when there are two different types of acceptor atoms and they have different covalency. Since the lone pairs are coming into action in this variant type. We have read that a lone pair will affect the overall geometry along with some alterations in the bond angle.
The lone pair will repel single or double covalent bonds formed adjacent to itself and thus it increases the bond angle from its side. On the other side, the bond angles between two single bonds will decrease. One lone pair is in the plane coming out from the screen, the other lone pair is placed on the screen entering into the screen, and thus two single bonds are in the same plane of the screen.
This variant is the deformed state of tetrahedral geometry and thus it is called the bent geometry. The usual bond angle of 109.6 degrees is not the same and it is reduced to 104.5 degrees.
- When there is no central atom: – P4 (Tetraphosphorus) is the three-dimensional molecule that has no proper central atom but still, it possesses the tetrahedral geometry. It is an inorganic molecule and natural state of being of phosphorus atoms. There are four phosphorus atoms occupying the vertex point in the tetrahedral geometry.
Each phosphorus atom is bonded to the remaining three different phosphorus atoms therefore, it has no specific central atom.
Tetrahedrane is another example of this variant of tetrahedral shape. Four carbon and hydrogen atoms bonded with a single atom of each other. One carbon is bonded to one hydrogen and other chains of the carbon atom. C – C – C is the structural representation of this variant.
The bond angle in this variant is 60 degrees and this also explains the torsional strain between atoms.
Conclusion
The tetrahedral geometry is a widely found geometry in the molecules, and it also has different bond angles. The tetrahedral shape is formed when four atoms in their elemental form join together by covalent bonding. By the word “tetrahedral”, we can get a slight idea about the meaning of this term. “Tetra” means the number four, and the word “hedral” depicts a solid face. When we combine the meaning of both these terms, we get to know that tetrahedral means a solid that has four faces. You learned what the tetrahedral shape of molecules is, why it is a three-dimensional molecule and the effect on a bond angle when lone pairs come into play.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out













