Introduction
Two or more atoms are joined together to produce chemical compounds. When the overall energy of the combination is smaller than that of the energy of the isolated atoms, the compound is viable. A chemical bond is implied by the bound state, which suggests a net attractive force between the atoms.
Types of chemical bonding:
The ways by which a chemical bonding can take place are mentioned below:
- Ionic bond: An ionic link is formed when all of the electrons in a molecule have been exchanged.
- Covalent bond: A covalent bond is formed by sharing of electrons.
- Hydrogen bond: A hydrogen atom joins several of the most electronegative atoms, including oxygen, nitrogen, and fluorine
- Van der Waal forces of attraction: Atoms are attracted to one another and form bonds.
Types of covalent bonding
The covalent bond can be one of three forms depending on the number of electron pairs.
Single covalent bond:
Just one pair of electrons is shared in between linked atoms to form a single covalent bond.
In the outermost quantum energy level, a chlorine atom has seven electrons. Two chlorine atoms combine to produce the covalent chlorine molecule, according to Lewis.
Each atom contributes electrons to form a pair that is shared by both atoms through covalent chemical bonding.
Multiple covalent bonds:
Lewis’s portrayal of numerous covalent molecular bonds was also expanded. Multiple bonds are made up of double and triple covalent bonds.
When two or three electron pairs are shared by two or three atoms, these types of bonds are produced. Bonding in oxygen, carbon dioxide, and ethylene molecules, for example, is produced by numerous covalent bonds. The triple covalent bond can be found in the acetylene molecule.
Sigma bond:
Sigma bonds (bonds) are a form of covalently chemical bond in chemistry.
The language and tools of symmetry groups are most precisely defined for diatomic molecules when it comes to sigma bonding.
A sigma-bond is symmetrical with respect to rotation about the bond axis in this formal method.
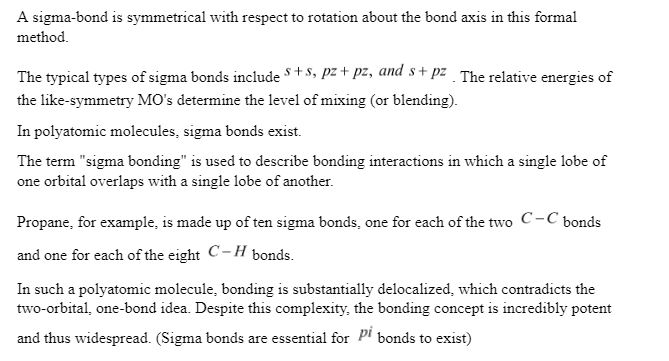
Sigma Bond Formation:
A sigma link is a covalent bond created by collinear or coaxial overlapping of an atomic orbital in a line of internuclear axis. Because orbitals are asymmetric, they can overlap on any side. As a result, overlap always results in a sigma bond. orbitals must be aligned along the internuclear axis to establish a sigma bond. They’re made up of s-s overlaps, such as, and overlaps, such as . and overlap, for example,
sigma bond’s characteristics
- Head-on-head overlapping of hybrid orbitals forms the sigma bond.
- The sigma bond is extremely strong and long-lasting.
- When two atoms or molecules engage with one another, the initial step is the development of a sigma bond.
- Typically, the sigma bond is represented by the sign.
- Sigma bonds form in all alkanes, alkenes, and alkynes.
Conclusion
The preponderance of bonding activity can be explained by the interaction between two distinct electrostatic forces. The electrons of an atom or ion are pulled to its positive charge (which contains protons), as well as the nuclei of nearby atoms. When chemical bonds are created, species that participate in them are more stable, usually because they have an imbalance of charge (more or fewer electrons than protons) or because their valence electrons do not fill or half-fill electron orbitals.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out















