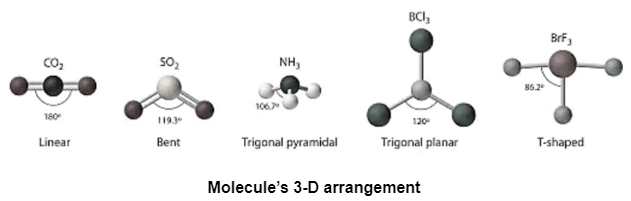
To determine the molecule’s shape the chemist performs VSEPR(valence shell electron pair repulsion) theory. It is based on the idea of increasing the distance between the electrons of shells so the molecule can stay stable. But this theory may fails with isoelectronic species electrons having the same number of molecules, elements and ions. Also during geometrical representation, you can take the help of VSEPR. Through VSEPR you can have a molecule’s 3-D arrangement which shows Molecular Geometry(polyatomic ion), and Electron Pair Geometry(electron pairs that are near to the central atom of polyatomic ion).
In chemistry, not just the formula fails to give the accuracy but also the many theories fail; this is why the limitations are present in every possible assumption.
Let’s Discuss the limitations
VSEPR is one of the great tools to monitor the shape of a molecule but there are still some limitations that are giving small doubts about it.
Isoelectronic Species
When valence electrons are having the same amount for two molecules this means it is an Isoelectronic Species. In this type of case, tellurium heptafluoride and iodine heptafluoride negatively charged ions are present, here VSEPR will be given the assumption of both having the shape of pentagonal bipyramidal. But in reality, you will see this shape of iodine heptafluoride only.
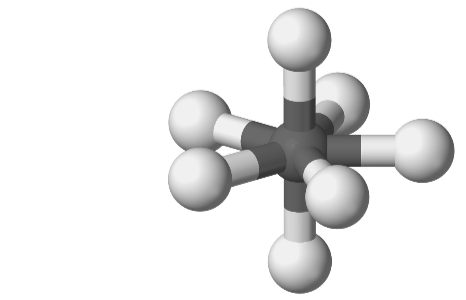
For heptafluoride, there will be a deviation from the above-given shape and that can’t be determined/presented by the VSEPR. So here the proof presents the VSEPR theory completely depends on the lone pair and bond pair. But even after having the same amount of electrons the isoelectronic species will have a different shape.
Transition metals
Transition metals are those metals that are not known for shape determination because of their presence in the atomic. Yes, they are having a large number of electrons but still, they will not show you the exact shape to determine the molecule. Due to changes in sizes some pairs will remain lone pairs which may affect the determining process. It shows that there is no bond between the two molecules which are leading to no pair effect. For example, [BrF6]- , [SeCl6]2- and [TeCl6]2- were predicted that they will pair up to show the geometry of pentagonal bipyramidal.
But due to the inactivity of electron pairs, the stereochemical no pairing effect of the molecules was found octahedral on a regular basis. In easy terms, it means that those who are not paired up are found to be not much active and because of that pairing up act goes in vain to justify the theory. Here the effect is almost similar to Isoelectronic Species.
Others
In group 2 elements i.e. Alkaline earth when they are examined according to the VSEPR theory here from 6 elements 4 are found to be pairing but the other 2 are not pairing. These group 2 elements include Beryllium (Be), Calcium (Ca), Strontium (Sr), Radium (Ra) Magnesium (Mg), and Barium(ba). These compounds are found to be volatile and they are surely producing the vapours. The VSEPR theory completely depends on the interaction but when there is no interaction you may find yourself getting an inaccurate result for your determination.
Quick Notes
- As per VSEPR theory, you can’t predict the shape geometrically of metals that are in transition.
- The theory fails in the Isoelectronic Species always when all the electrons are showing the same numbers.
- For lone pairs which are inactive, you can’t do anything even with the help of VSEPR theory.
- There will be ignorance of electrons who are having an orbital movement that is bound to the shape of the molecule.
- You will not get the exact angle of bonds for determining the geometrical shape of the actual molecule of transition metals.
Even if the VSEPR theory is not giving the exact result it is better to make use of it till you get the edge where you will not be getting an exact result. So just ease your further you can simply take the help of other trusted theories but VSEPR theory is surely one of the best to see the molecule shape. The theory is proven for normal movements mainly but when there are drastic changes where pairing up is not easy at that time you cant see the exact shape of the molecule.
Conclusion
Remember the VSEPR theory is totally based on lone pair to lone pair, bond pair to bond pair at last lone pair to bond pair only for bond pair to lone pair interaction you can’t rely on this theory. VSEPR theory does not consider the orbital movement of electrons and electrons’ interaction in the shell of atoms. This means the theory is ignoring the big factor which is helping in pairing the molecule to provide its shape geometrically.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out













