The Carius halogen technique is a quantitative method for determining halogens in chemical compounds in analytical chemistry.
In a furnace, a fixed quantity of an organic compound is heated in the mere presence of silver nitrate placed in a hard glass tube called a carius tube. The compound’s carbon and hydrogen are oxidised to carbon dioxide and water. The presence of halogen results in the formation of silver halide (AgX). It is filtered, rinsed, dried, and weighed before being used.
This chemical test is as effective for determining sulphur levels without the use of silver nitrate. With the addition of barium chloride, the sulphuric acid intermediate generated by the interaction of sulphur with fuming nitric acid transforms into insoluble barium sulphate. The addition of nitric acid serves to oxidise carbon and hydrogen. Nitric acid in concentrated form solely oxidises iodine to iodic acid and has no effect on other halogens. Iodine oxidation by concentrated nitric acid occurs only at extremely high temperatures.
Quantitative analysis
Quantitative analysis is a type of analysis that may be used to figure out how many elements or molecules are created during a reaction. Carbon and hydrogen make up organic molecules. Oxygen, nitrogen, phosphorus, sulphur, and halogens are among the elements found in them.
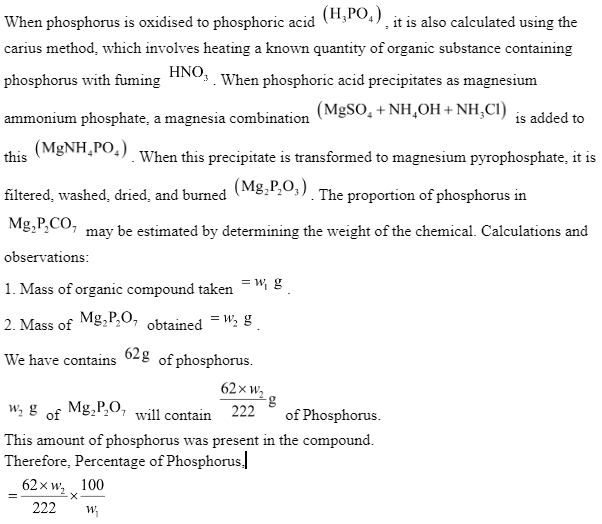
Estimation of Phosphorus
Carius Method
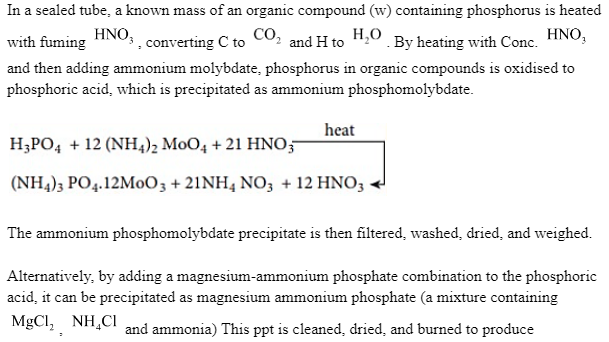
magnesium pyrophosphate, which is then cleaned, dried, and weighed. The following is the chain of events that occurs.
Calculations:
The proportion of P is computed using the mass of the organic component and the quantity of ammonium phosphomolybdate or magnesium pyrophosphate produced.
Mass of organic compound is ‘w’ gram.
Weight of ammonium phosphomolybdate = X gram
Weight of magnesium pyrophosphate = Y gram
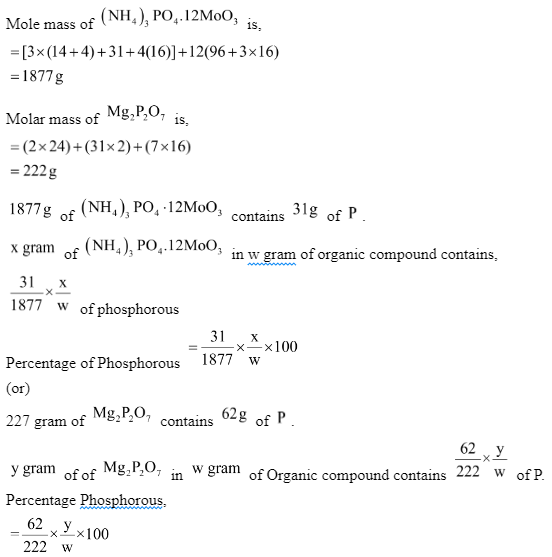
Solved Example

Note: The Carius Method is an alternate method for estimating phosphorus that involves boiling phosphoric acid with strong nitric acid and then adding ammonium molybdate to precipitate ammonium phosphomolybdate. This approach may also be used to calculate sulphur concentrations. The presence of halogens in organic compounds may also be detected since halogens generate sodium halide when they fuse with sodium metal, which is easily removed and recognised.
The mass of phosphor is calculated by multiplying the mass of the precipitate produced by the mass of a known organic constituent. The precipitate must be thoroughly cleaned, filtered, dried, and weighed.
Conclusion
The Carius technique involves heating an organic compound in a sealed tube with silver nitrate in strong nitric acid to determine the quantity of sulphur and halogens present. Silver sulphide and halides are precipitated, separated, and weighed after the complex is decomposed. The Carius technique is used to calculate halogen.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out