Detection of elements in organic compounds
The detection of components contained in an organic compound is the first stage in its analysis. Carbon, hydrogen, and oxygen are the primary elements. They may also contain nitrogen, sulphur, and halogens in addition to these elements. Phosphorous. Metals such as Li, Mg, and Zn can be found in organometallic compounds.
Quantitative analysis
Quantitative analysis is a type of analysis that may be used to figure out how many elements or molecules are created during a reaction. Carbon and hydrogen make up organic molecules. Oxygen, nitrogen, phosphorus, sulphur, and halogens are among the elements found in them.
Carbon and Hydrogen in a Compound:
Combustion analysis
Combustion analysis is the most popular way of detecting how much carbon and hydrogen are contained in a molecule. Combustion analysis involves burning a substance in the presence of pure oxygen, which yields solely carbon dioxide and water as products.
The masses of carbon dioxide and water generated are measured, and the quantity of carbon and hydrogen contained in the original molecule may be simply approximated by comparing the original compound’s mass to the masses of carbon dioxide and water created. A bomb calorimeter is a piece of scientific equipment that is commonly used for this sort of experiment.
Detection of carbon and hydrogen
There is no need to test for carbon if the chemical under inquiry is organic. This test is only used to determine whether or not a chemical is organic. Except for a few molecules such as and . All organic compounds include hydrogen. The following common test confirms the existence of both of these components.
Copper oxide test
Grinding is used to combine the organic component with about three times its weight in dry copper oxide. After that, the mixture is transferred to a hard glass test tube with a bent delivery tube. The other end is dipped in lime water in a different test tube. The mixture is rapidly heated, and the following reaction occurs.
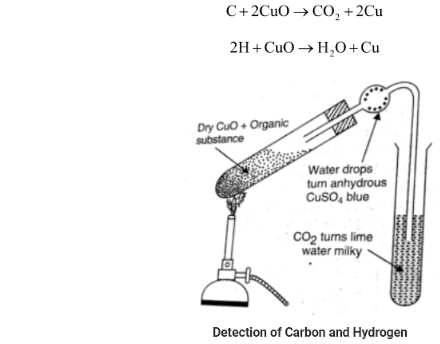
As a result, any carbon present is oxidised to CO2, turning lime water milky. If there is also hydrogen present, it will be oxidised to water, which will condense into little droplets on the test tube’s holder wall and inside the bulb. Anhydrous CuSO4 collects water, which turns the anhydrous CuSO4 blue. The presence of C and H in the compound is therefore confirmed.
Note: Test for carbon and hydrogen: A carbon and hydrogen-containing substance is heated in the presence of CuO in a dry test tube. The carbon is converted to CO2 while the hydrogen is converted to H2O. The H2O gas turns the anhydrous copper sulphate blue and the CO2 gas turns the lime water milky. The presence of carbon and hydrogen is confirmed by this inference.
Conclusion:
It is unnecessary to test for carbon if the chemical under study is organic. The purpose of this test is to determine whether or not a given substance is organic. All organic molecules include hydrogen, with the exception of a few chemicals like CCI4 and CS2. Copper oxide is a common test that can establish the existence of both of these components.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out











