Nitrous acid is a weak and monobasic acid. It has the ability to act as both oxidising and reducing agent. Nitrous acid is unstable because it breaks down rapidly into nitric acid (HNO3) and nitric oxide (NO). When nitrous acid is treated with amines, it produces diazonium salts which are used in azo coupling reactions of azo dyes in the dye industry. Nitrous acid is a very reliable laboratory agent as it is not very toxic.
Nitrous Acid
It is found in two forms called ‘syn’ and ‘anti’ in the gas phase. At room temperature, the ‘anti form’ is found to be more prominent than syn. The ‘anti-form is more stable as per infrared spectroscopy measurements. In the lower atmosphere, when nitric oxide reacts with water, nitrous acid is formed naturally. The molecular formula of nitrous acid is HNO2, and its molar mass is 47.013 g/mol.
Its colour appears to be pale blue, and the acid dissociation constant (pKa) is 3.15. The density of nitrous acid is 1g/ml. Melting point is only known in solution or in gas. It is non-flammable. It is weakly soluble in water. Nitrous acid needs to be prepared freshly as it is unstable in its free form and found in a liquid state most of the time. It can donate a proton (H+) to a base in an acid-base reaction, thus called monobasic acid. It forms salts when reacted with bases.
Chemical name (HNO2)
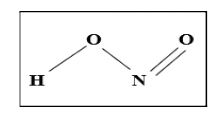
The structure of nitrous acid contains two oxygen atoms to a nitrogen atom, one bonded with a single bond and the other with a double bond. It is a planar molecule. The rotation of this structure is limited to the O-N-O bond because of the double bond characteristic. In the gas phase, the single-bonded oxygen binds with hydrogen to form cis and trans isomers.
Oxidation
Oxidation is where the chemical compound, atom or ion loses one or more electrons in a chemical reaction, and this leads to an increase in the oxidation state. The compound losing electrons will get oxidised, and it does not mean that oxygen is always involved in the reaction. But the addition of oxygen to the reactant may cause a loss of electrons. The compound undergoing a reduction reaction loses the hydrogen molecule.
For example– 1) Ethanal is produced when ethanol is oxidised.
CH3CH2OH → CH3CHO
2) The reaction between hydrogen and fluorine gas produces hydrofluoric acid, where the hydrogen is oxidised, and fluorine is reduced.
H2 + F2 → 2 HF
The two half-reactions taking place are-
H2 → 2H+ + 2e–
F2 + 2e– → 2F–
Thermodynamic reaction control and Kinetic reaction Control
- When a reaction is under thermodynamic control, the reactants and products in a reaction condition can be brought to equilibrium and the stability of the product formed is related to the amount of product formed. The major product in this condition is always under thermodynamic reaction control.
- In kinetic reaction control, the equilibrium cannot be achieved as the reactions that require a lot of energy are irreversible. Under kinetic control, the product with a low transition state requires low energy and is made faster. Thus, the major product in this reaction condition is kinetically controlled.
Oxidation of Nitrous Acid
The oxidation reaction of nitrous acid is under kinetic reaction control as the reactants are oxidised faster to form the product.
For example- Dilute nitrous acid can oxidise iodide into iodine but dilute nitric acid cannot.
I2 + 2e− → 2I− Eo = +0.54 V
NO−3 + 3H+ + 2e− → HNO2 + H2O
HNO2 + H+ + e− → NO + H2O
Here we can see that nitric acid is a strong oxidising agent, but based on the fact that dilute nitrous acid can oxidise I– to I2 faster than dilute nitric acid.
Conclusion
Nitrous acid is a weak acid and has been used in many industries to prepare other chemicals and is used as a solvent/ reagent for these reactions. It is present in the Liebermann reagent (A mixture of potassium nitrate and concentrated sulphuric acid) as an agent responsible for colour production (chromophore) for the detection of alkaloids. Dilute nitrous acid is found to be the faster oxidising agent as compared to dilute nitric acid.
Nitrous acid can destroy or eliminate the toxic and potentially hazardous explosive sodium azide. In the organic synthesis of the Sandmeyer reaction that synthesises aryl halides and aryl diazonium salts, these salts produced with the help of nitrous acid are widely used.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out