The generic geometry in which any type of molecule exists in nature is called its molecular geometry, which is determined by many theories from the valence bond theory or the VSEPR model theories. In chemistry, there are different types of structures drawn using three molecules; out of all those structures, trigonal planar is the one we will be studying through this article. VSEPR theory has played a significant role in predicting the structure of any covalently-bonded compounds formed by three molecules or more on the basis of the attraction-repulsion phenomenon that occurs between one atom or a lone pair or two close lone pairs. Before diving straight into the topic of the trigonal planar shape in a molecule, we will take you through the hierarchical order of concepts beginning from the molecular geometry and then into the trigonal shape of molecules.
What Is Molecular Geometry?
The term “molecular geometry” is used to define the arrangement of bond orientations developed between two, three, or more atoms together in order to form a single molecule. Since the word geometry is also involved, specific terminologies are also associated when we study molecular geometry, such as bond lengths, angle of torsional strain, bond angles, and covalency of each atom present in the molecule.
The bond angle, bond length, and torsional strain between two atoms of the same molecule don’t need to be equal.
Molecular geometry is a relevant topic to read before discussing any molecular structure to understand the actual reason why atoms have been shaped in a particular way and also their chemical properties such as polarity, reduction potential, magnetic properties, phase, or the state of matter, electrical conductivity, and biological activity.
The molecular geometry of any molecule depends on more than one factor; these factors are the strength of the central atom, the electronegativity or electropositivity power of the central atom, and the number of lone pairs present with the central outer atoms. The lone pairs of electrons contribute to depicting a fixed orientation or geometrical shape for any molecule.
Because, as the VSEPR theory says. The lone pairs of the electron, if placed close to each other, the lone pairs of the electron can repel the other lone pair and thereby modify the bond angle either by increasing it by more repulsion or decreasing it by loss aversion.
What Is The Molecular Geometry Of Trigonal Planar Shape Of Molecule?
The trigonal planar is the arrangement where a total of three molecules are combined to form the shape of an equilateral triangle, in which one atom is the central atom. All the four atoms combining the central atom lie in the same three-dimensional plane.
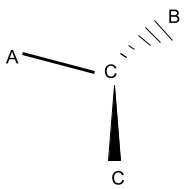
In this figure, C is the central atom with a covalency of three; therefore, three molecules have been attached to it, which are A, C, and B. Observing the closing, you’ll wonder what these dark lines are dotted lines portrayed in this figure.
The dark triangular badge is called the wedge, and the dotted lines are called the dashed lines. These two types of geometrical lines are not present in the molecule but are used for representation purposes.
The wedge is the line that signifies the covalent bond that is emerging out of the plane of the screen formed between one of the side atoms and the central atom. The dashed line represents the covalent bond going inwards into the screen formed between the other side atom and the central atom.
The hybridization of the trigonal planar molecule is sp2.
In trigonal planar geometry, the central atom possesses zero lone pairs.
Bond Angle In Trigonal Planar Shape Of Molecule
If bonded in a trigonal planar shape, the bond angle formed by three molecules will be decided by the lone pair associated with the central atom. Since there is no lone pair present in the central atom, the orientation of the three molecules generates an equilateral triangle structure in the normal state.
Let’s calculate the bond angles formed between two adjacent atoms in the trigonal planar shape of the geometry.
All the bond angles are equal, and we know the total sum of the angles around a point is 𝜋 degrees.
We divide 180 degrees into three parts to get the bond angle, i.e., 120 degrees.
If bonded in a trigonal planar shape, the bond angle formed by three molecules is 3𝜋/4 i.e. 120 degrees.
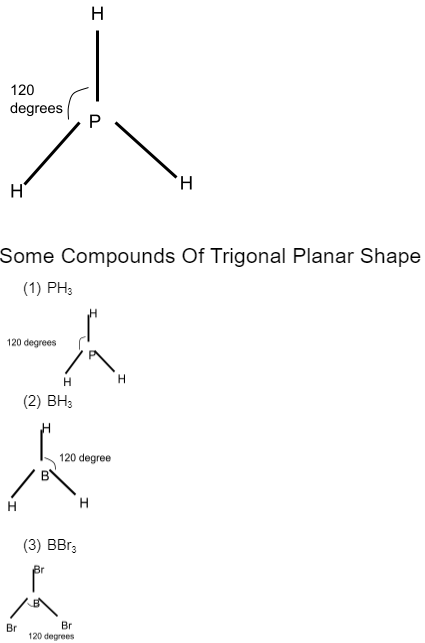
Conclusion
The molecular geometry of any molecule depends on more than one factor; these factors are the strength of the central atom, the electronegativity or electropositivity power of the central atom, and the number of lone pairs present with the central outer atoms. The lone pairs of electrons contribute to depicting a fixed orientation or geometrical shape for any molecule. Trigonal planar geometry is made from three molecules, and the type of molecule can be made from any trivalent atom. The equilateral triangle orientation tells that there is the minimum torsional strain in the trigonal planar shape of any molecule.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out











