Pyrosulfuric acid formula
Pyrosulfuric acid has the formula H2S2O7. The strong acid pyrosulfuric acid can be used to fume sulfuric acid. It is a Sulfur oxoacid. It’s made by combining Sulfur trioxide and sulfuric acid. It’s not as strong as sulfuric acid. Disulfuric acid is the name given to it. Because of its oily consistency and crystalline hue, it is also known as Oleum.
Structure of pyrosulfuric acid:
The bonds in the chemical compounds are shown in the structure of pyrosulfuric acid. The structural pyrosulfuric acid formula also shows the reaction that the chemical can undertake. It can be demonstrated as follows:
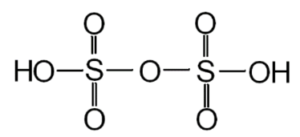
Pyrosulfuric acid preparation:
Sulfur trioxide and sulfuric acid are two chemical components required to make pyrosulfuric acid. It’s made by combining sulfur trioxide and sulfuric acid. According to the formula, H2SO4 + SO3 →H2S2O7
Also see:
Physical properties:
- It can exist in solid form, which makes it helpful for storing sulfuric acid in solid form.
- Disulfuric acid has a melting point of 360°C.
- It takes the shape of an oily, seething, dense substance.
- It is usually colorless.
- Color varies depending on purity grade, with a deep brown look occasionally.
- Persulfates are formed.
Chemical properties:
- Sulfo hydrogen sulfate is the IUPAC name for pyrosulfuric acid.
- Pyrosulfuric acid has a chemical compound weight of 178.129 g/mol.
- H2S2O7 is the chemical formula.
- The number of hydrogen bond donors is two.
- The number of hydrogen bond acceptors is seven.
- The number of hydrogen rotatable bonds is 7.
Applications ofpyrosulfuric acid:
- As a sulfating agent, it is utilized.
- It’s a type of explosive.
- It’s utilized in the dyeing process.
- In the petroleum refinery process, pyrosulfuric acid is employed.
- It is mostly used to store sulfuric acid in crystalline form.
- It can be used to make trinitrotoluene.
- It’s what’s utilized to make sulfuric acid.
Important chemistry formulas:
| Acetone formula | Citric acid formula |
| Urea formula | Butane formula |
| Photosynthesis formula | Magnesium oxide formula |
| Ethanol formula | Bond order formula |
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



