Potassium Sulphate Formula
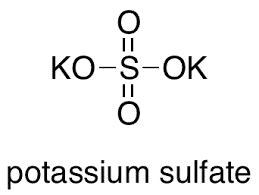
The Potassium Sulphate formula is commonly known as Dipotassium Sulphate formula or Sulphate Of Potash formula. This inorganic molecule comprises one sulphur atom, four oxygen atoms, and two potassium atoms.
Two of the four oxygen atoms bound to the sulphur atom are joined by double bonds, while the other two are connected by single bonds.
K2SO4 is the chemical or molecular formula for potassium sulphate.
Molecular Weight is equal to 174.259 gram/mole.
It comes in the form of colourless or white crystals, powder, or white granules. It has no odour and a bitter, gritty, saline flavour.
It can be dissolved in water but not in ethanol.
It can be obtained naturally from the minerals found in abundance in the Stassfurt salt.
It’s made by reacting potassium chloride with sulfuric acid to make sodium sulphate. Potassium bisulfate is formed as an intermediate in the exothermic process. It is commonly used in fertilisers as a potassium and sulphur source.
When you inhale K2SO4, you’ll get a sore throat and cough. It can cause redness and pain when it comes into contact with the eyes and skin. When this substance is consumed, it produces nausea, vomiting, abdominal pain, diarrhoea, and other symptoms.
Uses of Potassium Sulphate:
- Salt can be replaced with potassium sulphate
- It’s used to make pottery and glass, among other things
- As a flash reduction, it’s found in cannon propellant charges
- Soda blasting takes advantage of it
- Gypsum board is made with it
- Potassium aluminium sulphate is synthesised with it
- Gypsum cement is made with it
- As a flash suppressor, it’s employed in explosives
- It’s mostly used to fertilise tobacco, vegetables, and fruits
Production of Potassium Sulphate:
The following are the methods that were taken to obtain this compound:
- The mineral langbeinite is crushed
- It’s being cleaned
- Separating the mineral extraction
After that, the result is treated with an aqueous potassium chloride solution to separate the two portions of the double salt.
Synthetic potassium sulphate compounds can also be made. Potassium chloride is treated with raw sulfuric acid to achieve this.
Solved Examples:
1. Hydrolysis does not occur in K2SO4. Why?
K2SO4 is a strong acid and strong base salt. In a salt containing a strong base and a strong acid, neither the cation nor the anion undergoes hydrolysis.
2. How does a chemical process produce potassium sulphate?
Step 1: KCl + H2SO4 → HCl + KHSO4
Step 2: KCl + KHSO4 → HCl + K2SO4
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out