Carbanion pertains to a carbon atom with eight electrons in its valence shell with a negative charge. These eight electrons imply that a Carbanion is not electron-deficient, and these are electron donors sharing their electronic structure with amines. Carbanions are strong bases and face destabilisation with a group of donating electrons having bonded with an anionic centre consisting of eight electrons. Talking about the hybridisation of a Carbanion, it has an sp3 hybridisation. This article will deliver comprehensive knowledge with certain examples of the stability of carbanions in the following piece, along with a few examples asking you to arrange the following carbanions in decreasing order of stability.
Basics of Carbanions
Talking about the basics of carbanions, they are anions having an unshared pair with a negative charge, generally holding three substitutes. The geometry can be referred to as a trigonal pyramidal shape with a conjugate base in the first place.
R3C-H + B → R3C- + H-B
In the above equation, B reflects the base.
Carbanions are one of the various reactive agents in general organic chemistry.
Factors affecting the stability and reactivity of Carbanions
Now that you have become familiar with the basics of Carbanions. It’s time to move along and dive into the theoretical concepts of Carbanions in detail.
For starters, Carbanions are nucleophiles and below given are the factors responsible for the stability and reactivity of Carbanions:
- The charge on the carbon atom is stabilised under the inductive effect where adjacent electronegative atoms act as a stabilising agent
- The s-character defines the stability with direct proportionality
- Resonance also leads to greater stability, and it is particularly true in the case of aromaticity
Geometry of Carbanions
There are several theories like Bent’s rule and VSEPR that rule out the geometry of Carbanions. According to both these theories, the geometry of Carbanions is of bent, trigonal pyramidal and linear shapes. However, these shapes refer to various Carbanions, including aryl, alkyl, and alkynyl. With this, you now understand the concept of stability of carbanions and geometry. This gives you a basic idea of the Carbanions. Let us move on to the Carbon acids.
Introduction to Carbon acids
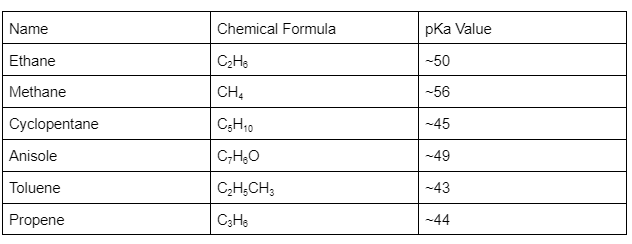
Molecules with a C-H bond tend to lose protons while forming a Carbanion. Acids having a C-H bond hold a corresponding value (pKa). Methane is not considered an acid, but it has a pKa value of 56.
On the other hand, acetic acid has a pKa value of just 4.76. The pKa value of a Carbanion is also determined the same way as stability. Here is the list of several carbon acids and their pKa values.
Carbanion Generation
The process of Carbanion generation involves organic reactions. There are several methods of Carbanions generation. Some of them are given below:
- Abstraction of protons
- Nucleophile addition to alkene
- Organometallic compounds formation
- Decarboxylation
Let us understand a few processes in brief
Abstraction of protons,
This includes the abstraction of protons and formation of an anion known as Carbanion.
R–H → R- + H+
Nucleophile addition to alkene
The attack of nucleophiles on any one carbon atom results in carbanions forming a negative charge on the left out carbon atom.
C=C → -C–C–Y
Chiral Carbanions
As discussed earlier, a Carbanion has a trigonal pyramid geometry. With this shape, it tends to show chiral properties.
As you know, the inversion of the trigonal pyramid shape can show racemisation, which is the same as the case of nitrogen inversion. The concept of chiral Carbanions dates back to 1950 when a reaction of sec-butyllithium with chiral 2-iodooctane was done to prove it. The reaction was assisted with petroleum ether at -70 degrees Celsius.
Conclusion
A carbanion refers to trivalent carbon with an anionic nature. Carbanions possess electron density concentration at the carbon atom, which is negatively charged. These Carbanions also act as reaction intermediates, with a vital function in several organic chemistry reactions. This concept of Carbanions started when the first Carbanions were discovered in a reaction of benzoin condensation. This article discusses carbanions’ stability and encapsulates questions that include practice to arrange the following carbanions in decreasing order of stability.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out