Physics is an ever-evolving subject. New developments keep on taking place which helps us gain deeper insight into the subject and the structure of the world, how the world is built. Atomic and nuclear physics is the field that deals with the deeper study of the atom structure, atomic nuclei and nuclear reactions. A very large part of nuclear physics takes its inspiration from particle physics.
Prerequisites for Further Exploration
- The world is constituted of the combinations of fundamental or subatomic particles, which forms the basis of the matter as a whole
- A typical atom is composed of two parts; atomic nucleus and subatomic cloud
- The number of protons and the number of neutrons present in an atom determines the properties or nature of its nucleus.
- The Standard Model in physics theoretically summarises the fundamental particles and its interactions.
- Nuclear decay takes place when an unstable atom loses energy and emits ionising radiation.
- The process through which two nuclear particles interact with each other to emit gamma rays is termed a nuclear reaction. Every particle reacts differently so separate studies are to be conducted for every particle to study the interaction of nuclear
Use of Atomic and Nuclear Physics
The knowledge of nuclear and atomic physics is essential for engineers working in nuclear reactors. In modern days, nuclear physics has found its way into several societal applications and is paving the way for new and advanced technology and innovations.
Atomic Physics
Atomic physics is the study of the atom structure and deals with atoms as an isolated system of electrons and the atomic nucleus. This field of study deals with the structure of atoms, the arrangement of electrons around the nucleus and the procedure by how the arrangements undergo several changes. The study provides the base for quantum physics, a celebrated cornerstone in modern physics.
Following concepts form the base of atomic physics:
- Thomson’s Experiment to find the charge/mass ratio
A combination of electric and magnetic fields was used by Thomson in 1896 to estimate the charge/mass ratio of a particle. J.J Thomson observed the deflection of the cathode rays due to the electrical field as he experimented on the cathode ray by controlling and balancing the effect of both magnetic and electrical fields. This experiment gave the charge/mass ratio as well.
Important formulas to remember:

The formula used when after both electronic field E and the magnetic field B are applied mutually perpendicular to the electron beam and the electron beam does not deflect is
- Millikan’s Oil Drop Experiment
In 1909, the scientist Milikan tried to measure the charge of electrons by balancing the gravitational and electric forces on the tiny suspended droplets of oil. After further experimentations, he concluded that in the presence of a light source, the velocity of the droplets can be observed from a microscope and timing the droplets as they travelled the predetermined distance of the experimentation. As the electric forces are controlled, knowing the electric field can help determine the charge being carried by the droplet.
The important formula for calculating the charge:

- Photoelectric Effect
At the end of the 19th century, scientists discovered that after coming in contact with some light source, some metals emit electrons from the surface. This experiment was termed the photoelectric effect and the electrons emitted were known as photoelectrons. This theory was further improved by Einstein who stated that the ray of light should be perceived not only as a wave but as made up of tiny packets of energy. The frequency of the single-photon was proportional to the frequency of light.
The important formula for Einstein’s photoelectric equation

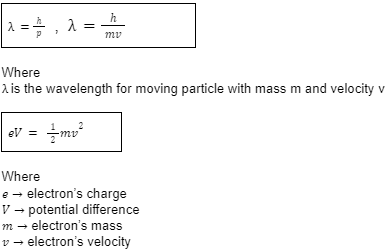
- De Broglie’s Hypothesis
In 1923, De Broglie hypothesised the wave-particle duality in which he stated that if light can display the behaviour of both wave and particles, then perhaps matter can also display such behaviour. While Broglie’s matter waves were rejected after further experimentation, evidence was found for the electron waves.
Important formulas:
Nuclear Physics
Nuclear physics is involved with the study of the protons, neutrons and their nuclear interactions. The primary research tool of nuclear physics involves the use of beams and particles directed towards nuclear targets. If there is a detection of recoiling particles or nuclear fragments, their direction and energies are analysed and studied to extract information about their nuclear and strong force.
Conclusion
Atomic and nuclear physics are vast subjects that further take inspiration from particle physics and form the base of one of the most celebrated cornerstones of modern-day physics, namely, quantum physics. The article summarises important concepts and experiments to revisit before diving deep into the topic.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out