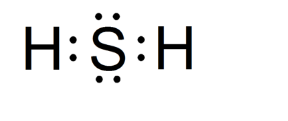The chemical compound hydrogen sulfide (H2S) has the chemical formula H2S. It comprises two hydrogen atoms and one sulfide atom. It’s a colourless gas with a distinct rotten egg stench that makes it easy to spot. It is somewhat denser than air and has the potential to be quite explosive. When hydrogen sulfide is burned with oxygen, it turns blue and produces sulfur dioxide and water. It is slightly water-soluble and functions as a mild acid.
H2S (hydrogen sulfide) is a colourless, dense gas. It has a distinct “rotten egg” odour at low concentrations and is exceedingly dangerous and even explosive at high doses. It is plentiful in nature, owing to the anaerobic decomposition of sulfur-containing organic materials. The sulfurization of petroleum fractions in oil refineries also produces a substantial amount of hydrogen sulfide. Let’s discuss the structure of hydrogen sulfide in detail!
Where is Hydrogen Sulfide found?
The structure of hydrogen sulfide, like many other harmful air pollutants, is formed when organic matter, such as plants or animals decomposes. It’s also emitted by sewage, manure and human and animal waste. Nature sulfur springs, like natural gas, are another widespread and somewhat infamous source for this odorous gas.
Many industrial production and refining processes, such as petroleum, pulp and paper, textiles, and food packaging, produce hydrogen sulfide as a byproduct.
Why is Hydrogen Sulfide a concern?
Hydrogen sulfide is a toxic air contaminant that is highly deadly. It is extremely corrosive when not properly diminished and can corrode metals, including stainless steel. The removal of H2S is the first stage in most abatement methods. It must be done to avoid damage to other equipment, including abatement technologies. It is highly flammable, explosive and extremely hazardous to human health. Even at very low concentrations, prolonged exposure can produce significant nausea, headaches, and eye discomfort.
Low concentration exposure might cause loss of appetite and exhaustion, and stomach trouble and inflammation. Loss of consciousness, permanent eye damage, respiratory difficulties, infection, and pulmonary oedema are more significant side effects that can occur at greater dosages.
H2S Lewis Structure
According to the H2S of the valence, electrons and nonbonding electron pairs participate in bond formation. Knowing the Lewis structure of a chemical compound is crucial since it provides information on all the substance’s other chemical properties.
Dots and lines in the illustration represent the electrons. Dots represent the electrons that do not participate in the bond formation. The lines, on the other hand, represent the compound’s bonds. The Octet Rule is used to create the structure. The Octet Rule stipulates that an element’s outer shell must have eight electrons to be stable.
H2S has the formula of an inorganic acid, yet the dissociation constant (Ka) for the first proton in an aqueous solution is just 1 x 10–7. The Ka of the second proton is a minuscule 1 x 10–12. Many inorganic cations [such as Cu(II), Pb(II), and Hg(II)] precipitate in H2S, making it suitable for qualitative identification of these ions.
Hybridization of H2S
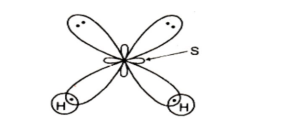
Follow these three easy procedures to understand the structure of hydrogen sulfide:
- Count how many atoms are linked to the centre atom. Only two hydrogen atoms are connected to the sulfur core atom.
- Count how many lone pairs there are on that central atom. So there are four lone pair electrons around sulfur, indicating that it has two lone pairs.
- Multiply the number of attached atoms to the centre atom by the number of lone pairs.
∴ 2 + 2 is equal to 4
As a result, four indicates that H2S hybridization is sp³.
What are the electron and molecular geometry of H2S?
Because the two lone pair electrons on the sulfur core atom oppose each other and nearby bonded pair electrons, these electron pairs (lone pair and bond pair) take the location where repulsion is the least and achieve stability (no other repulsion force exists in electron pairs).
As a result, H2S’s ultimate form or molecular geometry resembles a bent or V-shaped structure.
Is H2S polar or nonpolar? What is its dipole moment?
It is a well-known fact that polar molecules have some dipole moment due to unequal charge distribution. However, on the other hand, nonpolar molecules have an equal charge distribution and have zero dipole moment since the charges cancel out due to the molecule’s symmetrical form.
The molecule structure of Hydrogen Sulfide, electronegativity, and dipole moment of H2S determine its polarity.
Because the electronegativity of bonds (H-S) is less than 0.5, H2S is nonpolar. Hydrogen electronegativity is 2.20, sulfur electronegativity is 2.58, and their difference is 0.38, making H2S nonpolar.
Conclusion
Hydrogen sulfide (H2S) is a new neuromodulator that functions similarly to nitrogen oxide (NO) and carbon monoxide as a gasotransmitter (CO). H2S has cytoprotective properties and serves as a defensive mechanism in organisms as diverse as bacteria to mammals.
Related Links:
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out