Electrolytic refining is a technique used for the extraction and purification of metals by the process of electrolysis. Metals like copper, nickel, gold, lead, silver, and zinc can be purified using electrolytic refining.
Electrolysis
- The term electrolysis means the breakdown of substances through the use of electricity.
- It is a process that uses direct electric current to drive a non-spontaneous chemical reaction. In this process, electrolytes get decomposed by the passage of an electric current.
- Electrolysis is commercially important in separating elements from their naturally occurring sources like ores.
- In electrolysis, cations are reduced at the cathode, and anions are oxidised at the anode.
Principle of electrolytic refining of metals
In electrolytic refining, the anode is the impure metal to be purified, while the cathode is a thin strip of high purity metal. The electrolyte used in the solution is metal salt. When electric current passes through the electrolyte, pure metal from the anode dissolves in the electrolyte and gets deposited at the cathode. The insoluble impurities settle down at the bottom of the anode and are known as anode mud. The soluble impurities go back into the solution.
The electrolyte and other conditions must be appropriately selected so that anodic dissolution and deposition of the metal occurs with high efficiency, without impure metals getting transferred from the cathode to the anode.
Wherever necessary, additives should be added to the electrolyte to enforce correct behaviour at both electrodes. Chloride ion is a common additive to enhance dissolution.
Electrolytic refining of a few important metals
Copper
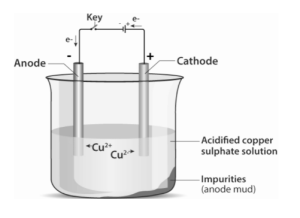
Impure copper is used as the anode, while a thin strip of highly pure copper is used as the cathode. Copper sulphate solution is used as the electrolyte. When electric current passes through the electrolyte, pure copper from the anode dissolves into the electrolyte, and the same amount of copper gets deposited at the cathode. Thus, the thickness of the cathode increases with time. The soluble impurities go into the solution, and the insoluble impurities settle down as anode mud. The anode mud contains antimony, selenium, tellurium, silver, gold, and platinum.
Reaction occurring at the cathode:
Cu2+ + 2e- —> Cu
Reaction occurring at the anode:
Cu – 2e- —> Cu2+
Properties and uses of copper:
- It has high electrical and thermal conductivity
- It can be moulded easily
- It has low toxicity
- It is a good conductor of electricity
- It is resistant to corrosion
- It has good soldering properties
Gold
Gold alloy is used as the anode, while a thin sheet of pure gold is used as the cathode. The electrolyte used is hydrochloric acid. When electric current passes through the electrolyte, it gets ionised, and the dissolved gold is transferred from the anode to the cathode, thereby increasing the gold’s purity at the cathode. This method is often referred to as Cycle Of Wohlwill.
Reaction occurring at the cathode:
Au+ + e- —> Au
Reaction occurring at the anode:
Au – e- —> Au+
Silver
Crude silver is used as the anode, while pure silver is used as the cathode. In this process, nitric acid bath is used as the electrolyte. Pure silver of about 99.9% is obtained in fine, needle-like crystal form at the cathode.
Reaction occurring at the cathode:
Ag+ + e- —> Ag
Reaction occurring at the anode:
Ag – e- —> Ag+
Zinc
Crude zinc is used as the anode, and a pure strip of zinc is used as the cathode. Acidified ZnSO4 is used as the electrolyte. When current passes through the electrolyte, pure zinc from the anode dissolves and gets deposited at the cathode. The soluble impurities go into the solution, while the insoluble impurities settle down as anode mud.
Reaction occurring at the cathode:
Zn2+ + 2e- —> Zn
The reaction occurring at the anode:
Zn – 2e- —> Zn2+
Nickel
In the electrolytic refining of nickel, pure Nickel is deposited at the cathode. Nickel sulphate solution or nickel chloride solution can be used as the electrolyte. In this case, anode mud contains Au, Ag, and Pt.
Reaction occurring at the cathode:
Ni2+ + 2e- —> Ni
Reaction occurring at the anode:
Ni – 2e- —> Ni2+
Conclusion
Electrolytic refining is the process of extraction and purification of metals through electrolysis. An impure metal is used as the anode, and a thin strip of pure metal is used as the cathode. A solution of metal salt is used as an electrolyte. The pure metal gets deposited at the cathode in the process, and insoluble impurities settle down as anode mud. Metals like copper, nickel, gold, lead, silver, and zinc can be purified using electrolytic refining.
Related Links:
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





