Organic chemistry is a significant branch of Chemistry that mainly deals with organic compounds’ structures, properties, and reactions with covalent bonds and carbon atoms. As the name suggests, hydrocarbons are a group of two chemical compounds called atoms carbon denoted as C and hydrogen H. Ancient chemists describe hydrocarbons as aromatic or aliphatic. Mainly, they are classified based on their properties and sources. However, today, hydrocarbons will be classified not just based on their origin but structure as well, which we will read here.
Today, we will briefly read about hydrocarbons, along with the classification and types of hydrocarbons in detail. So, let us get started!
A Brief Introduction to Hydrocarbons
Hydrocarbons can be described as organic compounds consisting of two major kinds of atoms — carbons and hydrogen. Generally, these are colourless gases with nearly no odour. Based on their types, hydrocarbons may possess simple or complex structures. Further, these are mainly classified into four major parts: alkenes, alkanes, alkynes, and aromatics.
Several hydrocarbons, such as propane and butane, are used for commercial fuel purposes such as LPG or Liquefied Petroleum Gas. Benzene, another significant aromatic hydrocarbon, is a raw material to make synthetic drugs. Another hydrocarbon called carotene is an organic pigment commonly found in carrots.
Classification of Hydrocarbons
Back in the day, hydrocarbons were majorly classified as either aromatic or aliphatic by several scientists. However, it was later discovered that the aliphatic hydrocarbon is formed through the chemical degradation of oils and fat. On the other hand, aromatic hydrocarbons mainly include ingredients formed by plant extract degradation. Here is the classification of hydrocarbons –
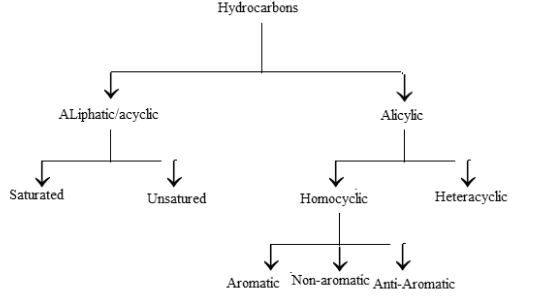
Types of Hydrocarbons
Hydrocarbons are divided into six categories. These include –
- Saturated hydrocarbons.
- Unsaturated hydrocarbons.
- Cycloalkanes.
- Aromatic hydrocarbons.
- Aliphatic hydrocarbons.
- Alicyclic hydrocarbons.
Let us discuss these in detail –
Saturated Hydrocarbons
In simple terms, saturated hydrocarbons are hydrocarbons in which every carbon-carbon bond is a single bond. Saturated hydrocarbons are hydrocarbons where all the carbon atoms bond closely to the other four atoms. Also, these are saturated, which means no carbon-carbon multiple bonds is present in these particular organic compounds. In saturated hydrocarbons, the carbon-carbon atoms and the carbon-hydrogen atoms are closely held together by a single bond that is the easiest hydrocarbon. Such hydrocarbons do not have either double or triple bonds.
Unsaturated Hydrocarbons
Unlike saturated hydrocarbons, unsaturated hydrocarbons are organic compounds solely made of hydrogen and carbon atoms. They consist of a triple and a double bond between the two adjacent carbon atoms. In unsaturated hydrocarbons, they consist of single, double, and even triple bonds between the carbon-carbon atoms. The triple-bonded compounds are referred to as alkynes, whereas the double-bonded compounds are referred to as alkenes. CnH2n-2 is alkynes’ general formula, and CnH2n is the formula for alkenes.
Cycloalkanes
Cycloalkanes are hydrocarbons that possess either one or more carbon rings. The hydrogen atoms are closely attached to these carbon rings. This ring forms because of the saturated nature of Cycloalkanes. They have three alkane compounds that form the ring. Some common examples of Cycloalkanes are cyclohexane, cycloheptane, cyclopentane, Cyclobutane, cyclooctane, etc.
Aromatic Hydrocarbons
Also known as arenes, aromatic hydrocarbons are compounds with at least one carbon ring. Aromatic compounds are chemical compounds that satisfy Huckel’s rule. They consist of planar molecules with conjugation in the ring format. These compounds have a name such as aromatics or arenes.
These compounds do not have a single or double molecular bond, instead, they have the company of pi-electron clouds in the delocalisation state. Aromatic hydrocarbons are the primary example of aromatic compounds bonding with the pi-electrons cloud. In aromatic hydrocarbons, many elements like Benzene; Toluene forms the sigma bond with planar ring conjugation.
Aliphatic Hydrocarbons
Aliphatic hydrocarbons have completely straight chain structures with no carbon rings in them. It consists of carbon and hydrogen atoms linked in chains through single, triple, or double bonds. At times, these chains may appear in non-aromatic structures.
Alicyclic Hydrocarbons
It is another type of hydrocarbon that has a ring structure in them. Sp, Sp2 or Sp3 can be the carbon atoms. It is common to see naturally occurring alicyclic hydrocarbons.
Comparison Between Saturated Hydrocarbon and Unsaturated Hydrocarbon
| Saturated Hydrocarbon | Unsaturated Hydrocarbon |
| In saturated hydrocarbons, all the carbon atoms are sp3 hybridised. | In unsaturated hydrocarbons, all the carbon atoms are sp2 or sp hybridised. |
| As compared to unsaturated hydrocarbons, saturated hydrocarbons have more hydrogen atoms. | As compared to saturated hydrocarbons, unsaturated hydrocarbons have fewer hydrogen atoms. |
| Common examples of saturated hydrocarbons are alkanes and cycloalkanes. | Common examples of unsaturated hydrocarbons are alkynes, aromatic hydrocarbons, and alkenes. |
| Saturated hydrocarbons possess low chemical reactivity. | Unsaturated hydrocarbons possess relatively high chemical reactivity. |
| Saturated hydrocarbons burn with a blue flame | Unsaturated hydrocarbons burn with a sooty flame. |
Conclusion
With this, we come to an end to our discussion of classification and types of hydrocarbons. Hydrocarbons are organic compounds made up of essential atoms called hydrogen and carbons. In everyday life, hydrocarbons play a significant role, and their study provides a deep insight into the properties and preparation of its functional groups.
Hydrocarbons are classified into several subparts and divided into six major parts: saturated, unsaturated, cycloalkanes, aromatic, aliphatic hydrocarbons, and alicyclic hydrocarbons, all of which we discussed in detail. Each is different from the other, and so are their formulas. At last, we quickly compared saturated and unsaturated hydrocarbons for ease of understanding. We hope you attained a better understanding of the classification and types of hydrocarbons.
Related Links:
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





