Tartaric Acid
Tartaric acid has the chemical formula C4H6O6 and is a carboxylic acid.
Tartaric acid is an organic acid present in a variety of vegetables and fruits, including bananas, grapes, citrus, and tamarinds. It’s also known as Racemic acid or 2,3-dihydroxysuccinic acid. It is utilized in the production of carbon dioxide. It’s a crystalline white diprotic aldaric acid that’s diprotic.
It is commonly used in the pharmaceutical industry. A high amount of tartaric acid can cause death or paralysis.
Properties of Tartaric Acid – C4H6O6
Tartaric Acid | C4H6O6 |
Molecular Weight/ Molar Mass | 150.087 g/mol |
Density | 1.79 g/mL |
Melting Point | 171 to 174 °C |
Boiling Point | 275 °C |
Structure of Tartaric Acid
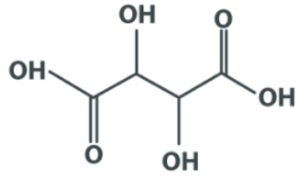
Applications:
- It’s used to make oral drugs taste better
- It’s a chelating agent for metal ions like magnesium and calcium
- It, along with baking soda, is used as a leavening agent in recipes
- It’s a type of antioxidant.
- It’s one among the most crucial acids in wine.
- It is occasionally used to cause vomiting and is used in dishes to give them a sour taste
- Silver mirrors are made from it
- It is utilized in the colouring of textiles in its ester form
- It is used in the tanning of leather and in the production of chocolates
- It is used as a food stabilizer in cream form
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




