Sodium Sulfide
Sodium sulfide is a compound with the chemical formula Na2S
They are colourless and when dissolved in water they become strongly alkaline solutions
When exposed to moist air, sodium sulfide and its hydrates release strong hydrogen sulfide that smells like rotten eggs
Industrially, sodium sulfide is produced by the carbon thermal reaction of sodium sulphate with charcoal
Na2S | Sodium sulfide |
Density | 1.86g/cm2 |
Molecular Weight/ Molar Mass | 78.0452g/mol |
Autoignition temperature | >480 oC ( 896 oF; 753 K) |
Melting Point | 1,176 oC (2,149 oF) |
Odour | Like rotten eggs |
Appearance | Anhydrous yellow crystalline solids |
pH | 10.4 |
Oxidation number | -2 |
Solubility | Insoluble in ether; slightly soluble in alcohol |
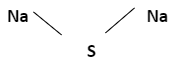
The chemical formula of Sodium Sulfide
Na2S
Chemical structure
Solved examples
What happens when sodium sulphide dissolves in water?
It forms the corresponding ions
Na2S + H2O → 2Na+ + HS– + OH–
Can sodium sulfide be easily oxidised when heated?
Yes, it can, when heated it generally forms sodium carbonate and sulfur dioxide
2Na2S + 3O2 + 2CO2 → 2Na2CO3 + 2SO2
On reacting with sulfur it forms polysulfides
2Na2S + S8 → 2Na2S5
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out